8.2a Reactions with acids and bases
Reactions with acids and bases:
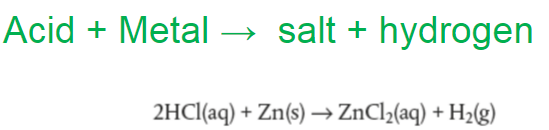
Can you figure out acid reacts with metal oxides ?
Acid reacts with metal hydroxides (usually a base)
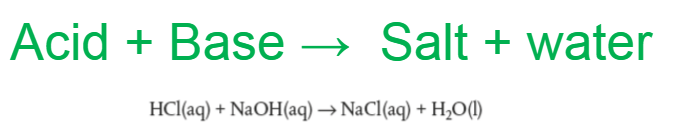
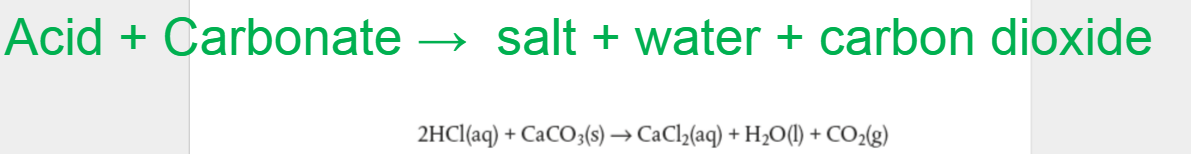
Can you figure out acid reacts with hydrogen carbonates?
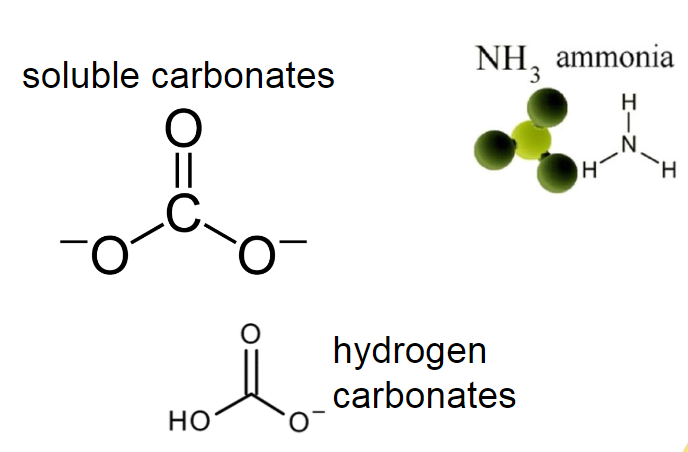
Bases which are not hydroxides: