8.2b Neutralization and indicators
Neutralization reaction
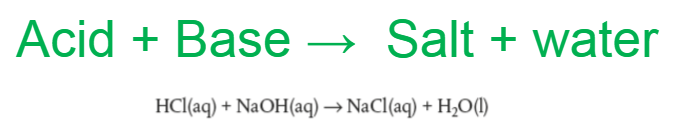
This is an exothermic reaction!!! So keep them separated
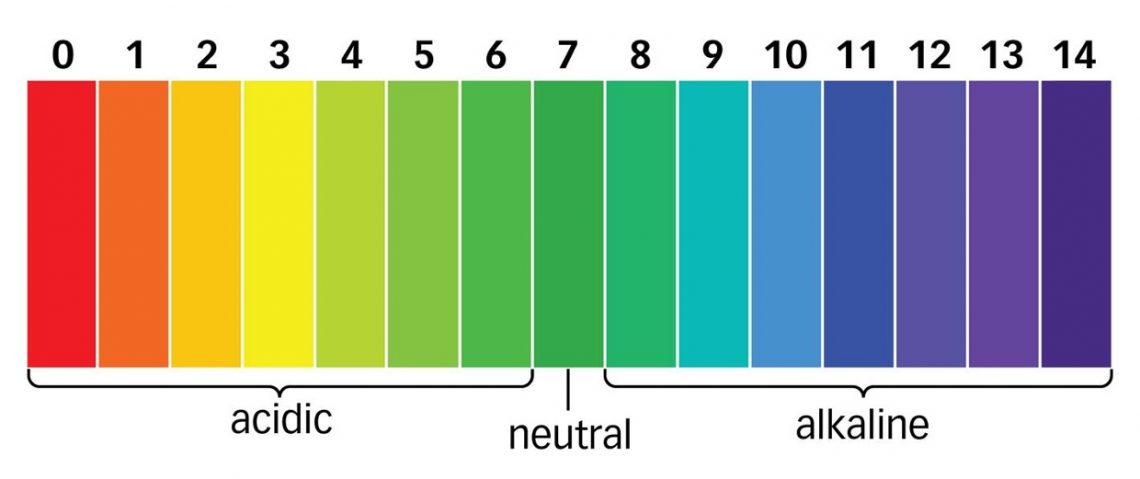
Indicators data booklet in section 22
act as chemical detectors by changing color to tell you the pH of the solution.


8.2b Neutralization and indicators
Neutralization reaction
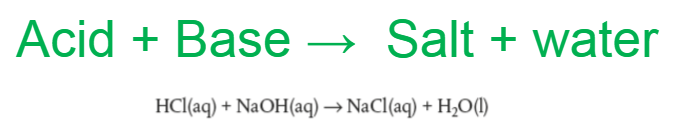
This is an exothermic reaction!!! So keep them separated
Indicators data booklet in section 22
act as chemical detectors by changing color to tell you the pH of the solution.


