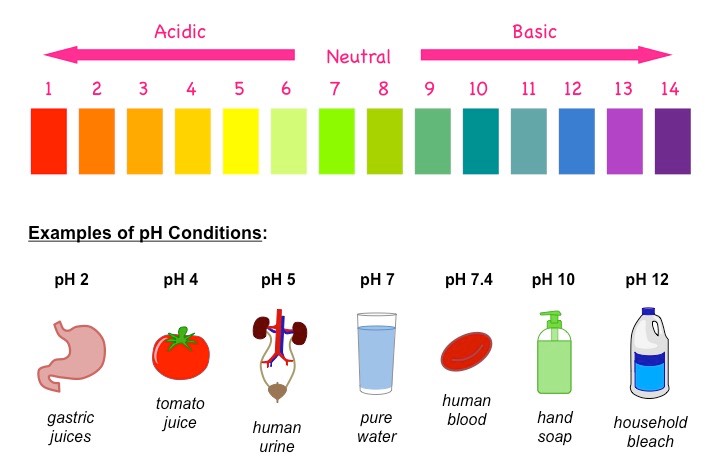
8.3a What it means?
The pH scale is an artificial scale used to distinguish between acid, neutral and basic/alkaline solutions.
pH is a log base of the concentration of protons
pH = − log[H+] and [H+] = 10−pH
Exercise
From a 1M solution of HCl and 1M solution of NaOH, make solutions of pH 2 to 10 with increment of 1. Use a pH meter for this task.









