8.3b Problems involving pH
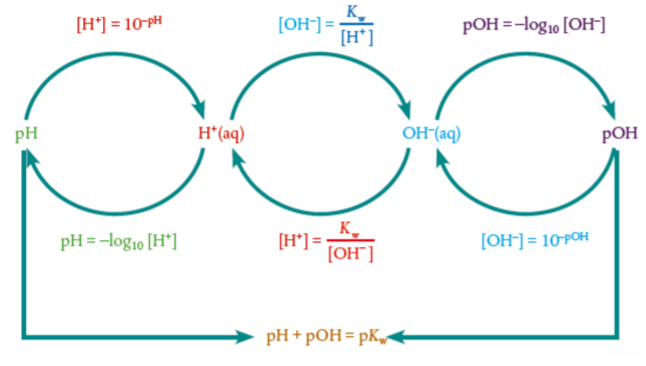
The ionic product constant and pH equations
Kw = [H+][OH−] = 10−14
pH = − log[H+]
How do you get pH of a base?
Example: Find pH of 1M NaOH
First method:
1M NaOH (strong base), thus [OH-] = 1M
Kw = [H+][OH−] = 10−14
So [H+] = 10−14 / [OH−] = 10−14 / 1M = 10−14
pH = − log[H+] = - log(10−14) = 14
Different way:
Likewise pOH = − log[OH-] = - log(1M) = 0
pH + pOH = 14
So pH = 14 - pOH = 14 - 0 = 14
Keep in mind inverse relationship between the two measures.