8.4a Ionization determines strength
section 21 in the data booklet for a list of weak acids and bases
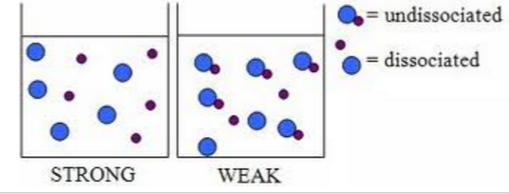
Ionization or dissociation determines strong or weak acid (same with base)
Strong acid ionization
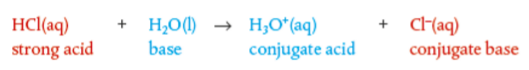
Weak acid ionization
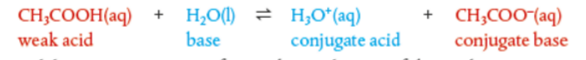
What is the different? Hint: look at the arrows.
What is the ionization for base?
NaOH + H2O --> Na+ + OH-
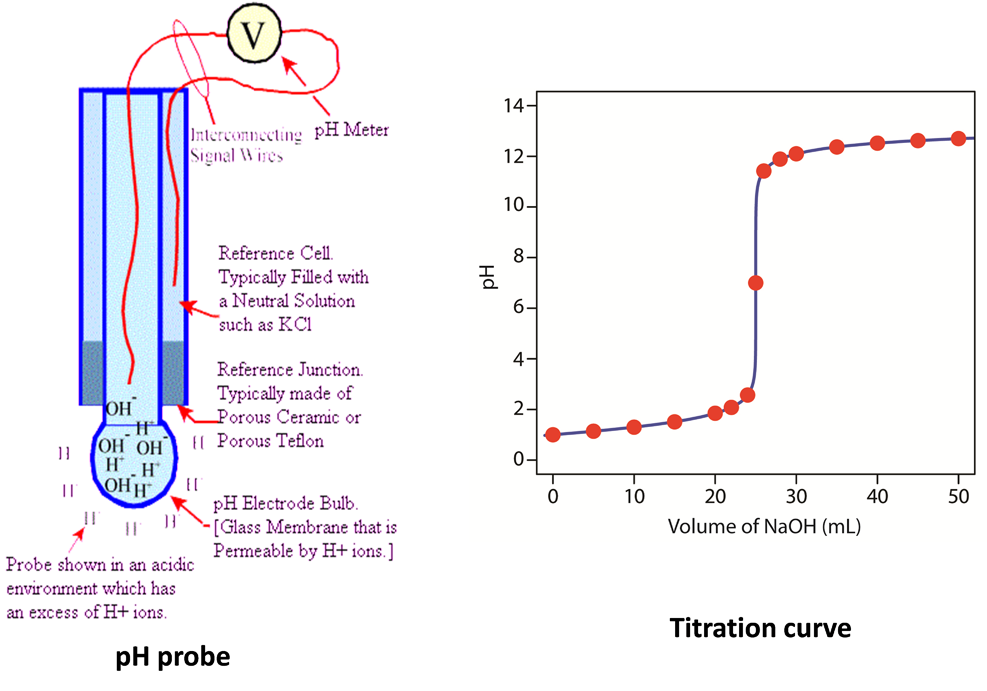
Use data loggers to investigate the strength of acid and bases
- The pH of weak acid is higher than pH of a strong acid of the same concentration.
- The more [H+], more conductive the aqueous solution is. Ie. The conductivity increase
- The electronic pH meter operates this way.