8.4c Rate of reaction and strength
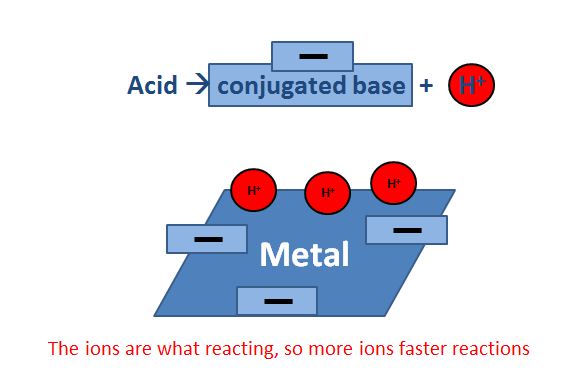
Three ways to measure strength of acid (or base): all based on the amount of ions
![Machine generated alternative text:1. Electrical conductivity — depends on the concentration of ionsso strong acids and bases will show higher conductivity thanweak acids and bases2. Rate of reaction — depends on [H] so stronger acids will havefaster reaction rates3. pH — the pH scale can be used directly to compare thestrengths of acids. Remember the higher the [H], the lowerthe pH value](http://ibmole.com/upload/files/images/Topic%208/8_4_4_2.png)
Concentration is very important
See video below. Is the acid used strong or weak acid? How does it have the effect?
For solutions of equal concentrations, how does strength affect the rate of reaction?









