8.5a Gases that cause acid rain
Memorize these gases
nitrogen or sulfur oxides dissolve in water to form HNO3, HNO2, H2SO4 and H2SO3
Note that CO2 is NOT causing acid rain because dissolved CO2 and has a pH of 5.6. Acid deposition (acid rain) has a pH below 5.6
Cause
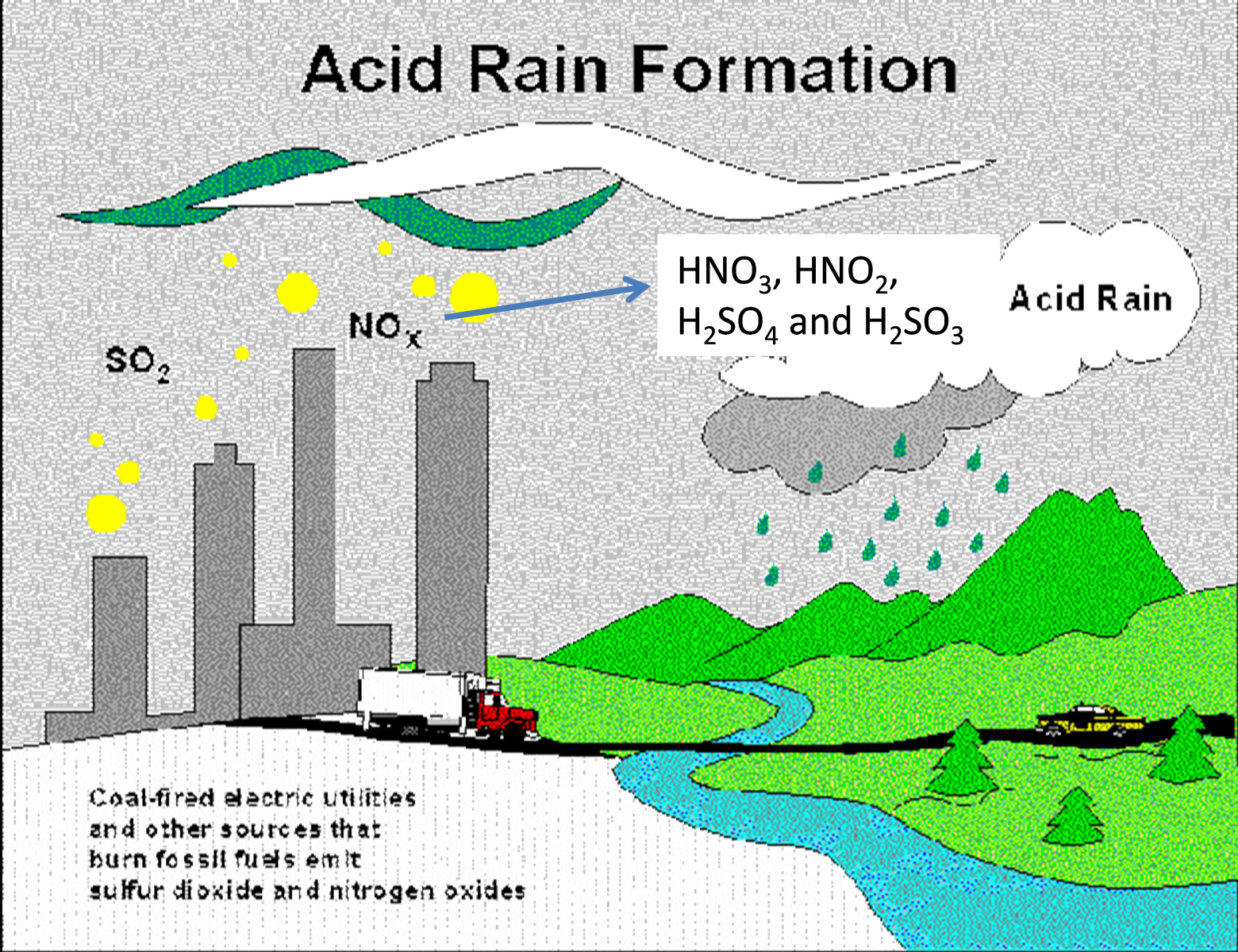
Write the equations that describe the combustion of sulfur and nitrogen to their oxides and the subsequent formation of HNO3, HNO2, H2SO4 and H2SO3
N2 + O2 --> 2NO
2NO + O2 --> 2NO2
2NO2 + H2O --> HNO3 + HNO2
Consequence

Write the equations for acid deposition with reactive metals and carbonates
How do we reduce it?










