4.1a Ionic bond
Ionic compounds consist of ions held together in lattice structures by ionic bonds.
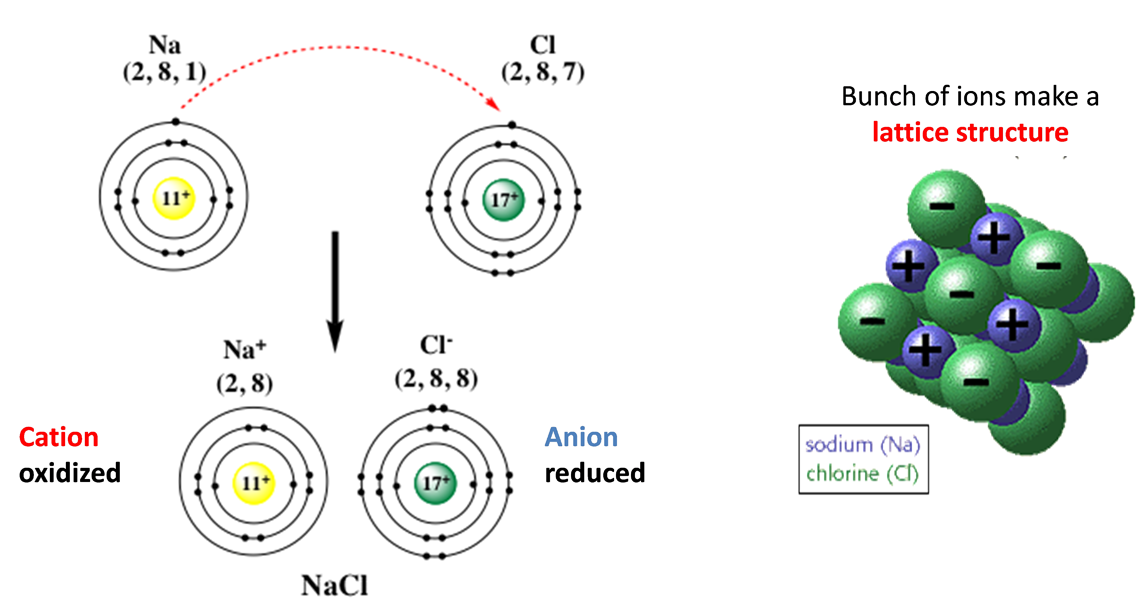
- An ion is a charged particle.
- The number of charges on an ion is equal to the number of electrons lost (positive ion) or gained (negative ion) by an atom.
- Metals lose electrons to form positive ions (cations); non-metals gain electrons to form negative ions (anions).
- The charge on an ion can usually be predicted from the group of the element in the Periodic Table; transition metal elements can form more than one ion.
- Ionic compounds consist of ions held together by forces of electrostatic attraction.
- Ionic compounds are electrically neutral, as they consist of a lattice in which the total number of positive charges is balanced by the total number of negative charges. The formula of the compound is expressed as its simplest ratio, e.g. the ions Xm+ and Yn– will form the compound XnYm.
- In the ionic lattice, each ion is surrounded by a fixed number of ions of the opposite charge, known as the coordination number.
Memorize the names of these common polyatomic ions
Note that the bonds within the polyatomic ions are covalent bonds!









