4.1b Characteristic of ionic compounds
Ionic compounds usually have these characteristics:
- high melting and boiling point
- more soluble in water than in non-polar solvents. (A few exception to this trend thou)
- They conduct electricity when molten or in aqueous solution but not when solid.
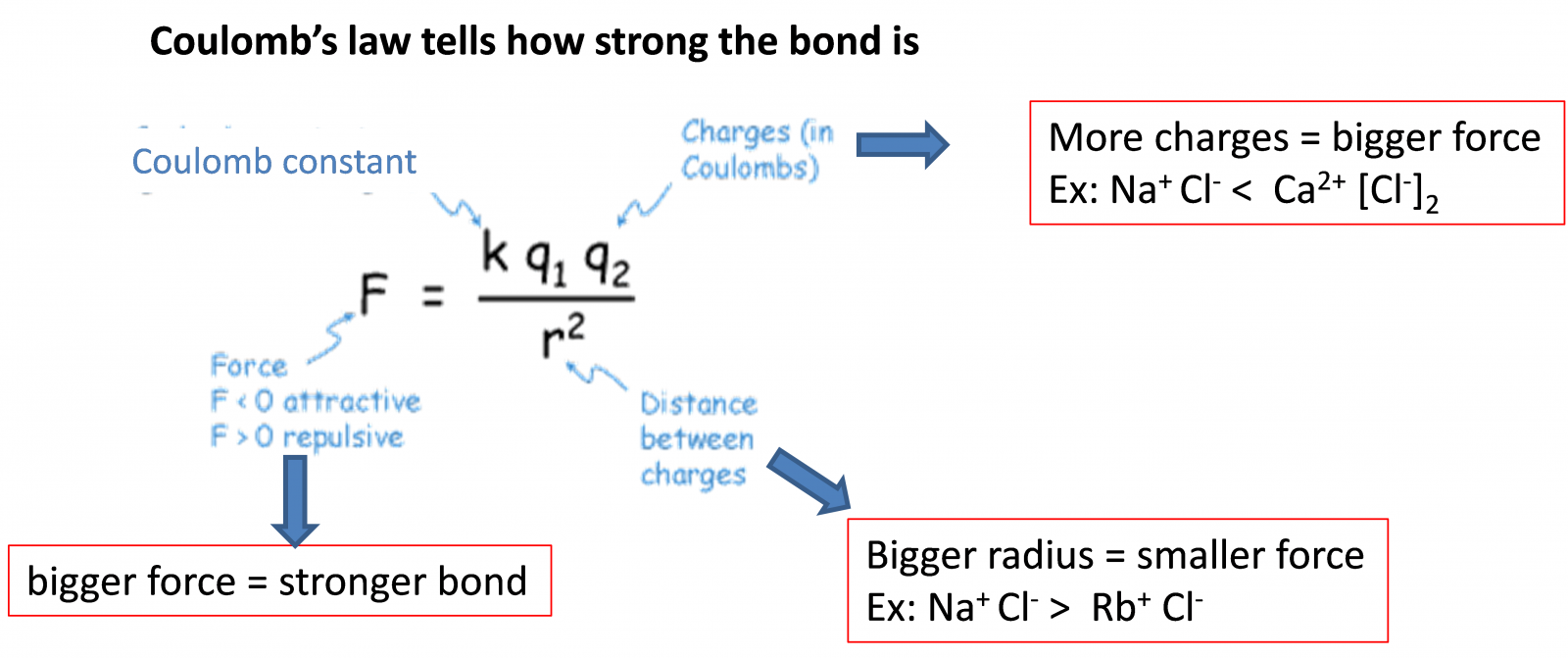
Physical properties of ionic compounds depend on how strong the ionic bond is.
Stronger bond = higher melting and boiling points = less volatility (less likely to vaporize) = more solubility (easy to dissolve) in water
In short, for stronger bond look for
1. More charges Eg. Ca2+ make stronger bond than Na+
2. Smaller atomic radius Eg. Na+ make stronger bond than Rb+
Why? (You don't have to know this following part but math makes sense to some people)