4.2 Covalent bonding
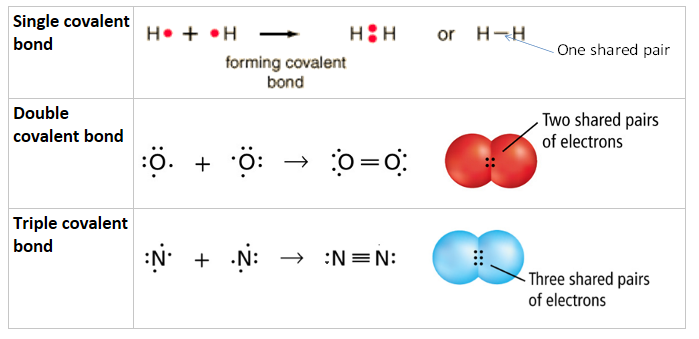
Molecular compounds form by the sharing of electrons.
Note that each line between atoms (like H-H) represents 2 electrons being shared, NOT one electron
Honestly, just watch the playlist below!!!
- A covalent bond is the electrostatic attraction between a pair of electrons and positively charged nuclei.
- A molecule is a group of atoms held together by covalent bonds.
- Two pairs of shared electrons = double bond.
- Three pairs of shared electrons = triple bond.
- Increasing number of bonds Þ shorter and stronger bonds.
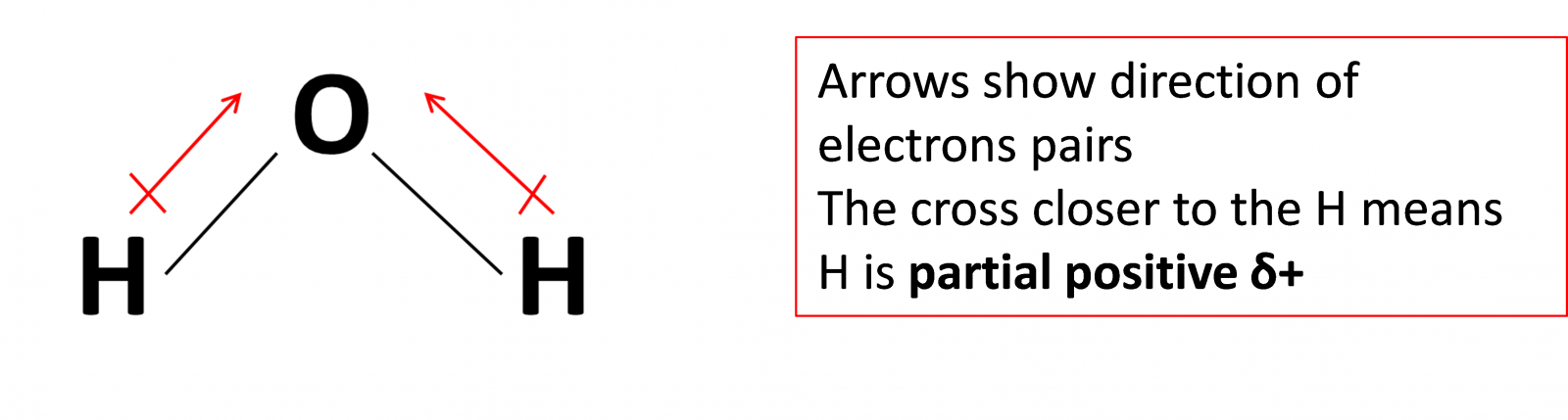
Polar or non-polar covalent bond?
- Polar bonds form when the two atoms bonded together have different electronegativity values.
Electronegativity is the ability to attract electron, use the Data booklet
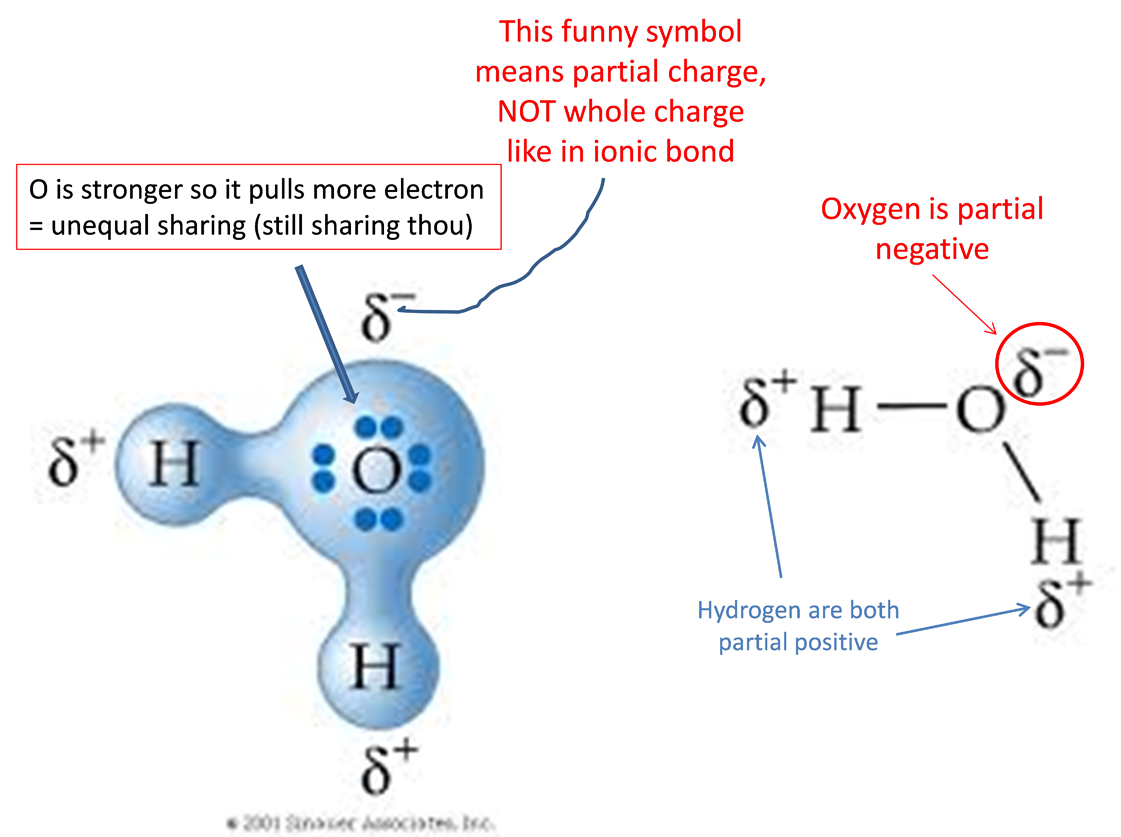
Dipole moment is another way to show pulling of electrons in a polar covalent bond