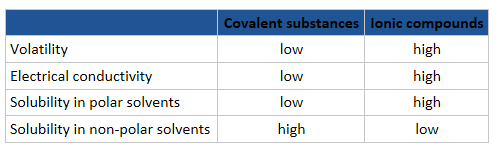
4.4b Covalent molecule - Physical characteristic
Physical properties depend on how atoms or molecules of the materials are stuck together in its structure (more below)
The physical properties of molecular substances result from different types of intermolecular forces between their molecules.
How do you compare the strength of intermolecular bonding?
- In order of strength:
London (dispersion) < dipole–dipole < hydrogen bonding
- To compare London: more electrons or more stacking = stronger bond
or more MW = stronger London. Example from OrganicChemTutor below.
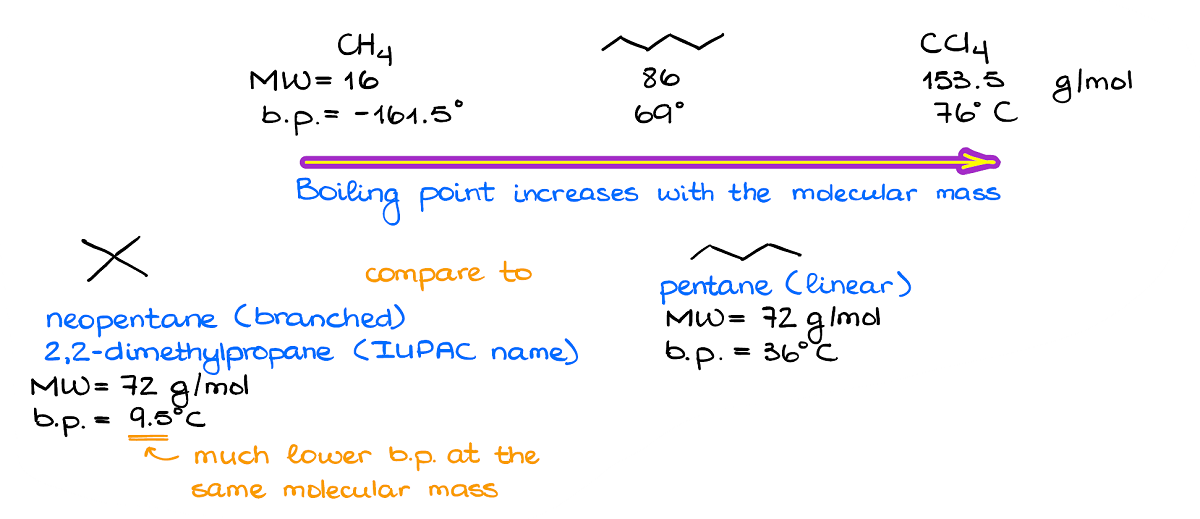
How does intermolecular bonding effect melting point and boiling point?
Stronger bond = higher melting and boiling points = less volatility (less likely to vaporize)
Solubility and conductivity
How do you know what will dissolve in water?
"like dissolve like": Polar substances are more soluble in water (which is polar) and less soluble in non-polar solvents.
Covalent compounds are generally not good electrical conductors, unless they are able to ionize in solution, e.g. HCl(aq). Why?