BIG idea: Physical properties of materials
What are physical properties? Properties associated with physical change (that’s cheating, you can’t use the same word in the definition!) OK, think about change that doesn’t alter the chemical formula of the material itself
- melting and boiling point. Example: ice melts into water, water evaporates into gas. H2O is still H2O, it does change into a different compound → physical
- Volatility = likely to evaporate
- Malleable (bendable), ductile (stretch out into string), brittle (easy to break)...
- Solubility in water or in non-polar solvents.
- Conduct electricity. This has to do with the number of delocalized electrons.
Physical properties depend on how atoms or molecules of the materials are stuck together in its structure (more below)
How do you compare physical properties of different materials?
Step 1: consider what force bring the structure together
Look at the intramolecular (ionic, covalent, or metallic)
Step 2:
- If ionic or metallic, physical properties depend on how strong the ionic/metallic bond is.
Stronger bond = higher melting and boiling points = less volatility (less likely to vaporize) = for ionic compound, more solubility (easy to dissolve) in water
In short, for stronger bond look for 1. More charge of the ions (higher charge density) and 2. Smaller ionic radii
Note: the material structure is held together by these bond, that’s why ionic compounds like NaCl and metals have high melting point, ie. solid at room temperature
- If covalent, proceed to step 3
Step 3 (for covalent only): consider is this material a giant covalent network or a molecule?
- If giant covalent network, physical properties depend on the geometry of the network. See https://ibmole.com/4-14-bonding/content-558
- If molecule, the force that holds the material together is Intermolecular force. When you boil the material, the molecules are separated. Only the intermolecular force is broken, the covalent bond (between the atoms) are not. This is why covalent molecule are usually gas at room temp (low boiling point).
To compare the strengths of different intermolecular force see https://ibmole.com/bonding-and-transition-metals/content-162 AND https://ibmole.com/bonding-and-transition-metals/content-163
From s1.1.1 - How is mixture different from compound?
Things (atoms or molecules) inside a mixture are not bonded to each others by ionic, covalent, or metallic bond. A compound must have atoms bonding to each other.
How are two types of mixtures differ?
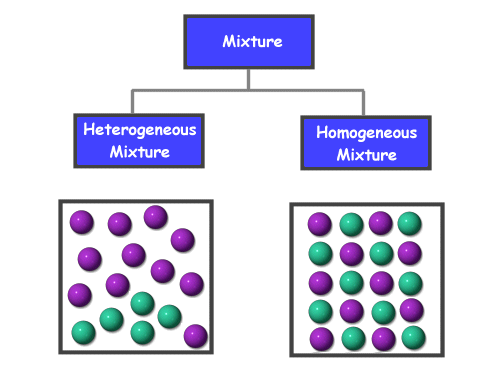
Examples:
Solutions (homogeneous) : salt and water
Alloys (homogeneous) : One example of an alloy is steel which is made from a mixture of iron and carbon
Suspensions (heterogeneous) : An example of a suspension is a mixture of water and sand
Colloids (heterogeneous): An example of a colloid is milk. Milk is a mixture of liquid butterfat globules dispersed and suspended in water.
How are mixtures get separated?
Solvation - dissolving a solute (the solid stuff) in a solvent (the liquid stuff, usually water)
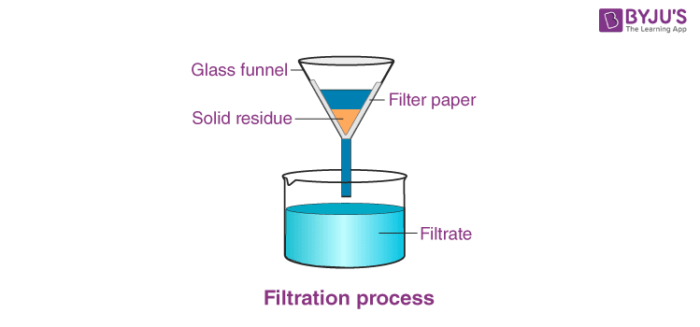
Filtration - filter out the solute that wasn't dissolved in the solution
Recrystallization - reduce temperature so that the solute (pure substance) will "fall out" of the supersaturated (too full) solution
Evaporation - heat up to make the solvent (liquid part) turn into gas, leaving just the solute (pure substance)
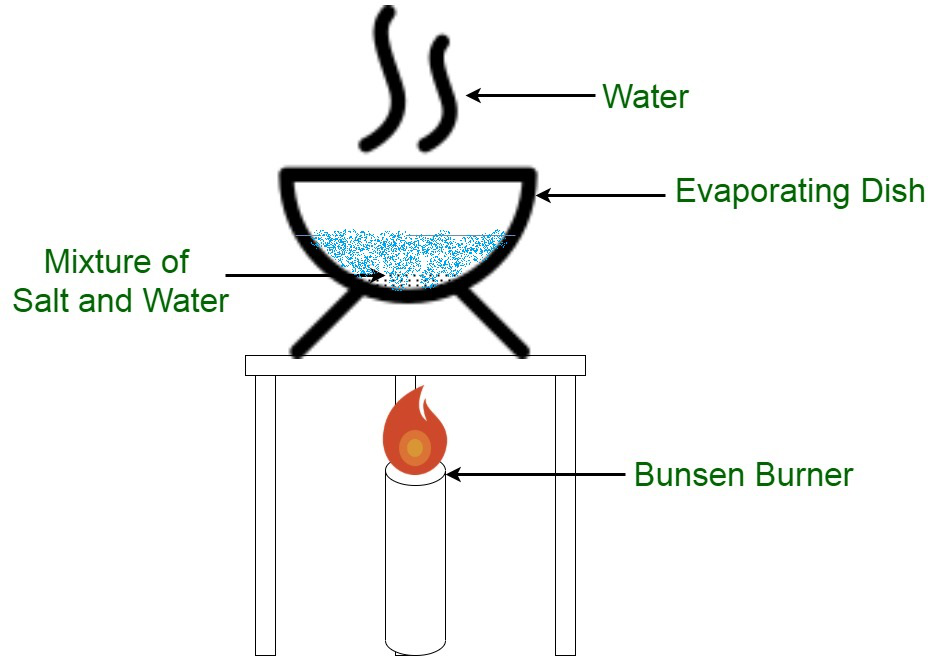
Usually solvation, filtration, and recrystallization/ or evaporation techniques are used together to separate the mixture
Distillation - separating the components of a liquid mixture through selective evaporation and condensation ie. a liquid evavoprate at lower temperature than the other liquid. Therefore, you can separate them.

Chromatography - separating components of a mixture using chromatography paper or resin ie. due to different properties of the components (mass, charge, solubility...), different parts of the mixture moves through the "special" paper or column of resin at different speed, thus you can separate them.










