5.1b Endothermic and exothermic reactions
Key things to memorize (more info below)
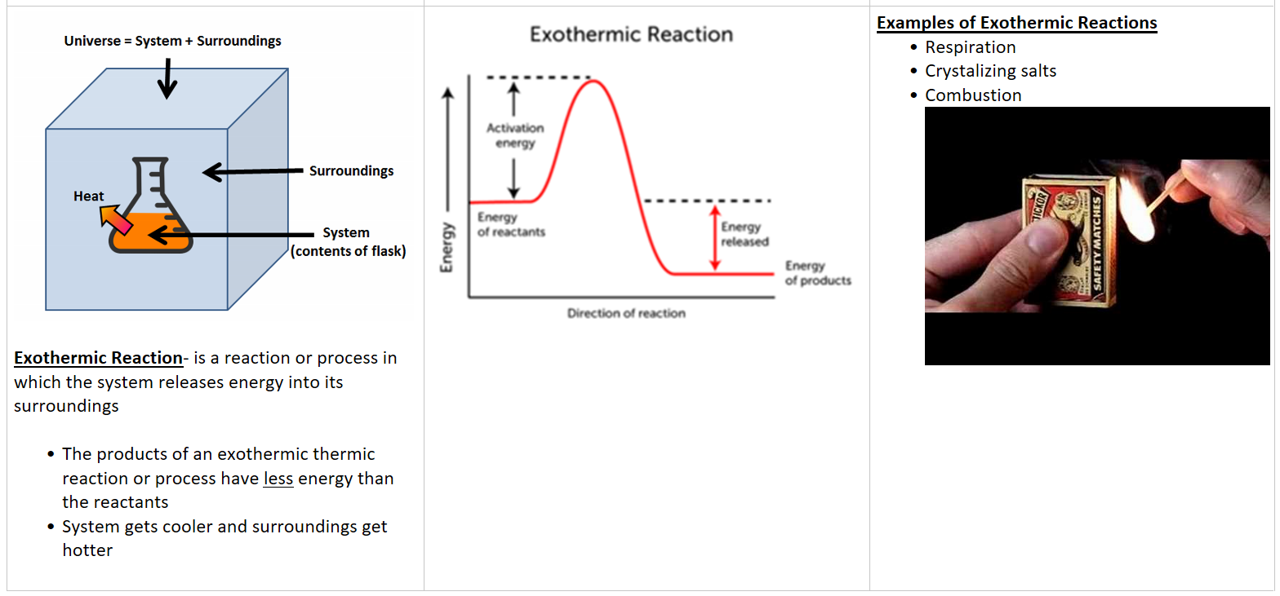
- Exothermic = energy go out = negative (-) sign
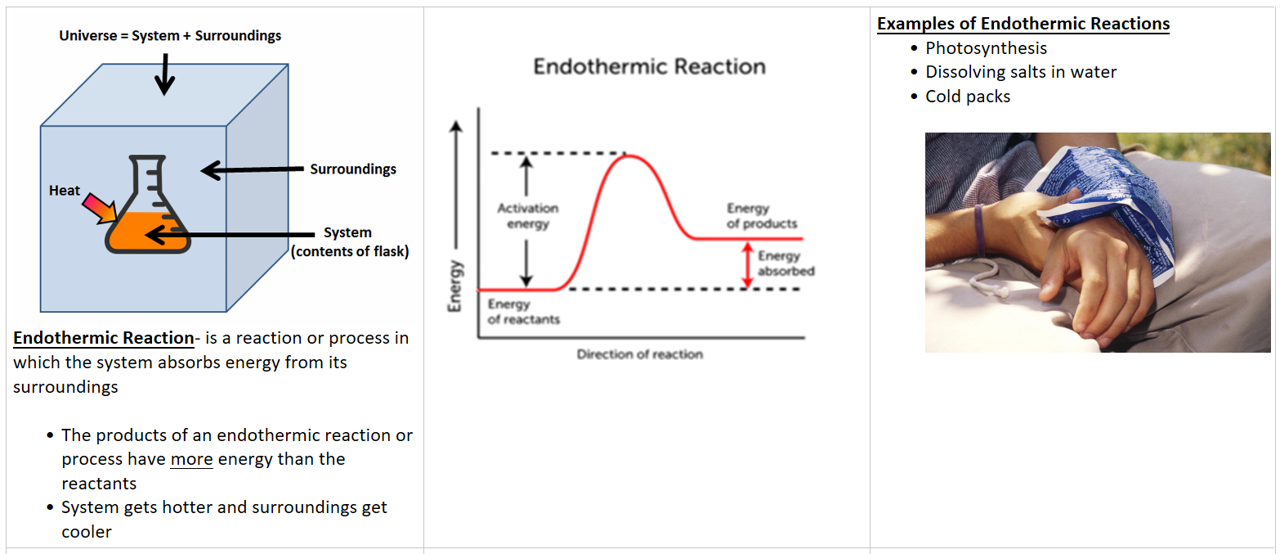
Endothermic = energy go in = positive (+) sign
Make your own memoric device to remember this. Eg. Korean pop group "exo" has a lot of antifans so exo is (-)
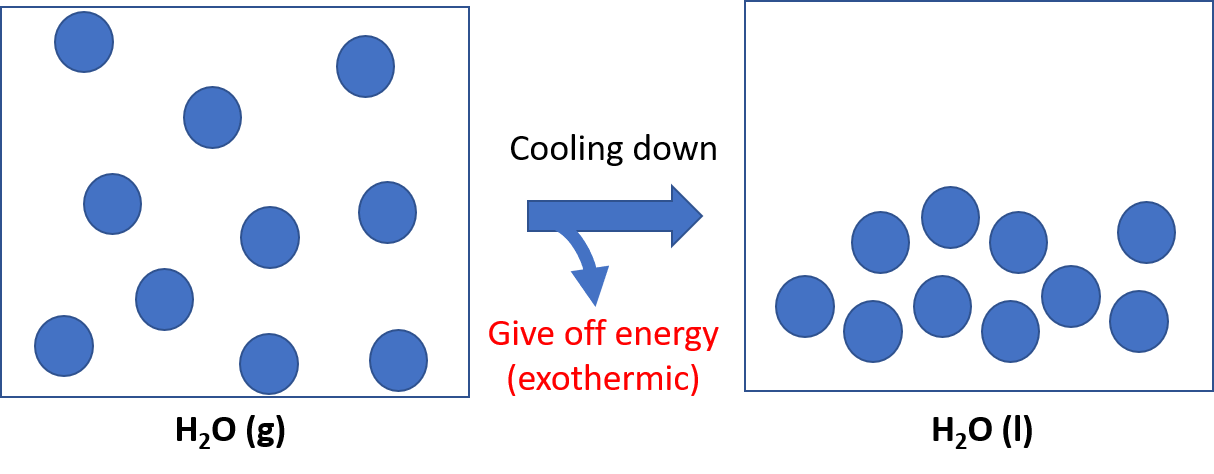
- Breaking apart = endothermic (because you have to put in energy)
Forming bond/ coming together = exothermic
Examples. H2O (g) --> H2O (l) exothermic because molecules are clumping together
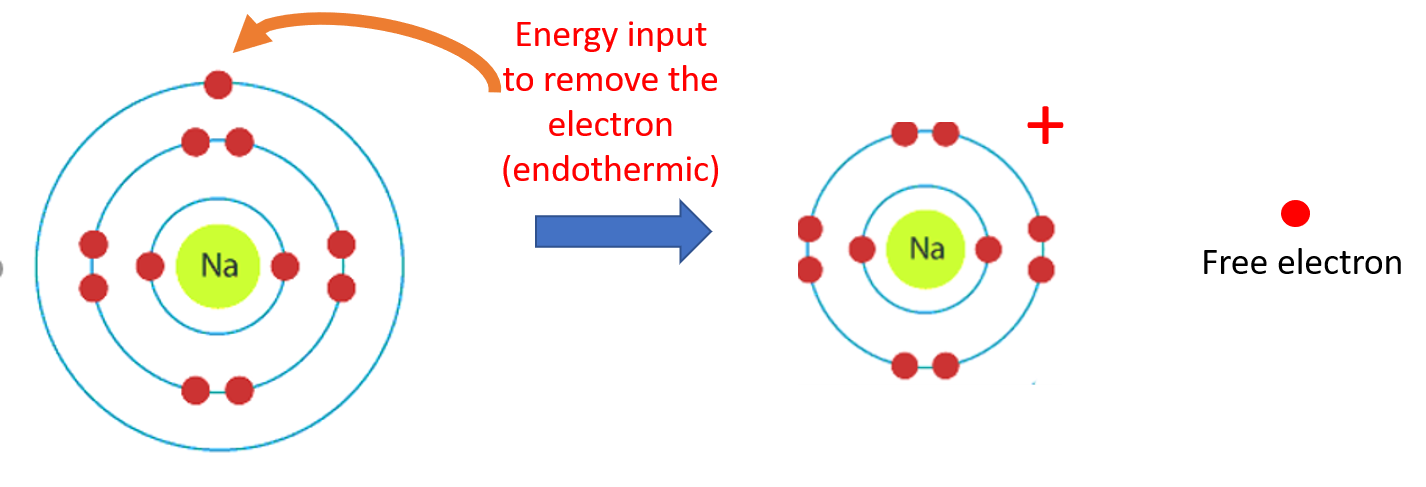
Na (g) --> Na+ (g) + e- endothermic because electron comes apart