5.1c Aqueous solution enthalpy change
Thermal equilibrium
• Temperature determines the direction that heat will transfer
○ Heat always moves from a hotter object to a cooler object
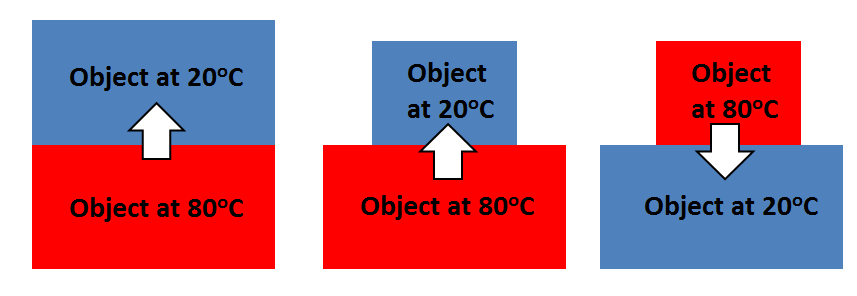
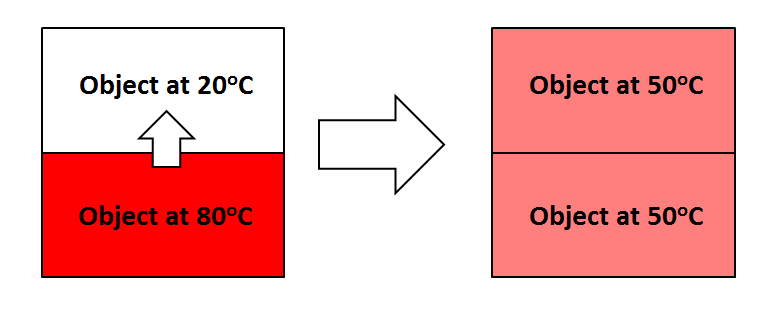
Thermal equilibrium-occurs when both objects reach the same temperature and the transfer of heat stops
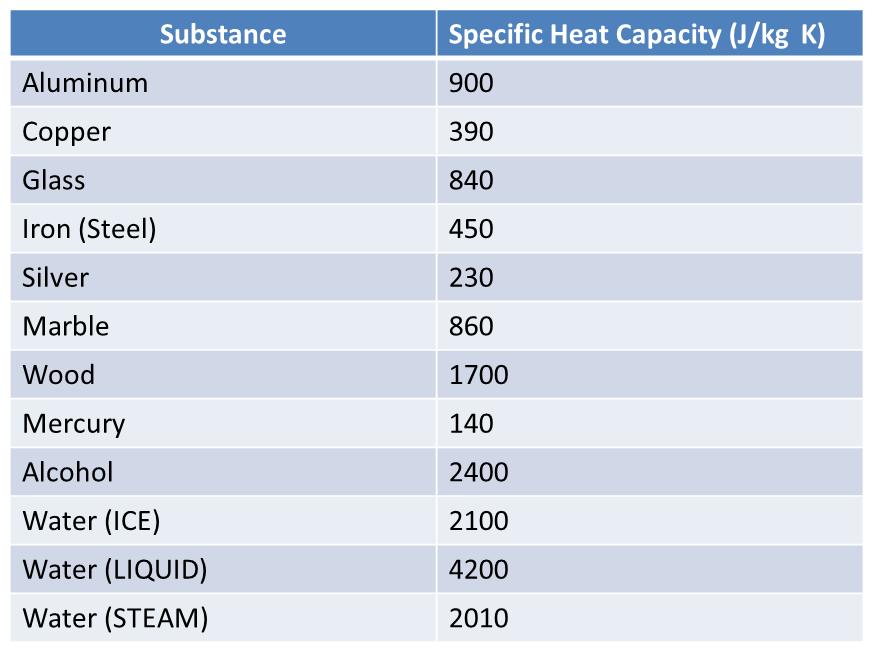
Specific Heat Capacity
Specific Heat Capacity(c) -is the energy that must be transferred to 1kg of substance to cause its temperature to increase by 1K.
c= Q/m∆T
Q => Heat in joules (J)
m => mass in kilograms (kg)
∆T => Change in temperature in (K)
• ∆T= T_final − T_inital
Different Materials and states of matter have different heat capacities
Example
If 840 J of heat is transferred to 1kg of iron, the temperature of the iron will be increased by 1K.









