5.1e Enthalpy change of a reaction
Standard condition. Symbol Ѳ
Standard state refers to the normal, most pure stable state of a substance measured at 100 kPa. Temperature is not a part of the definition of standard state, but 298 K is commonly used.
Example: standard state of CO2 is gas, of butter is solid
ΔHfѲ of CO2 is -393.5 kJ/mol
What is enthalpy change?
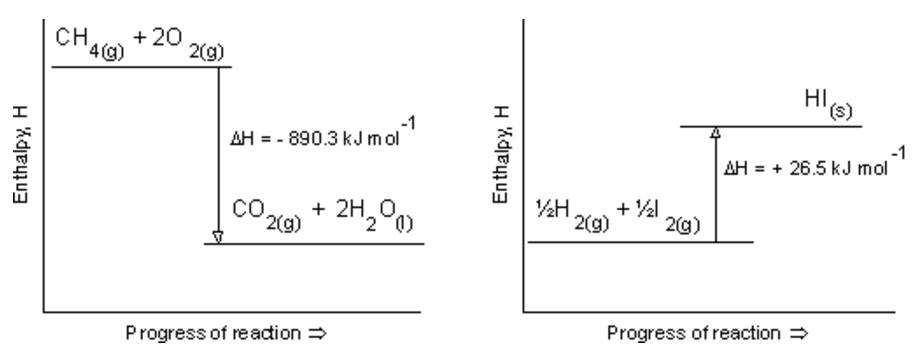
Note: there is NO UNIT on the y-axis because you cannot know what is the enthalpy of the reactants and products, BUT you can know the difference or change in enthalpy (∆H)
Standard enthalpy change of formation ΔHf
Is the energy change upon the formation of 1 mol of a substance at standard state.
ΔHfѲ = Σ(ΔHfѲ products) - Σ(ΔHfѲ reactants)
Example: 2 H2O --> 2 H2 + O2
ΔHfѲ = (2* enthalpy H2 + enth O2 ) - 2 * enthalpy H2O
= 0 - (-285.8 Kj/mol)*2 = +571.6 kJ
Standard enthalpy change of combustion ΔHc
Is the heat involve in burning of 1 mol of a substance.
Example:
2 H2 + O2 --> 2 H2O
ΔHcѲ = -286 KJ/mol
For this equation ΔHcѲ = - 572 KJ
Enthalpy change per mole
Example: above H20 example. To find enthalpy per mole. Divide enthalpy to the # of moles.









