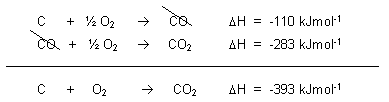5.2a Hess's law
State function: is a quantity whose value depends only on the state at the moment and NOT how the system gets to that state
Example: temperature, pressure, volume, etc… Enthalpy of a system is also a state function.
Hess's law make simple:
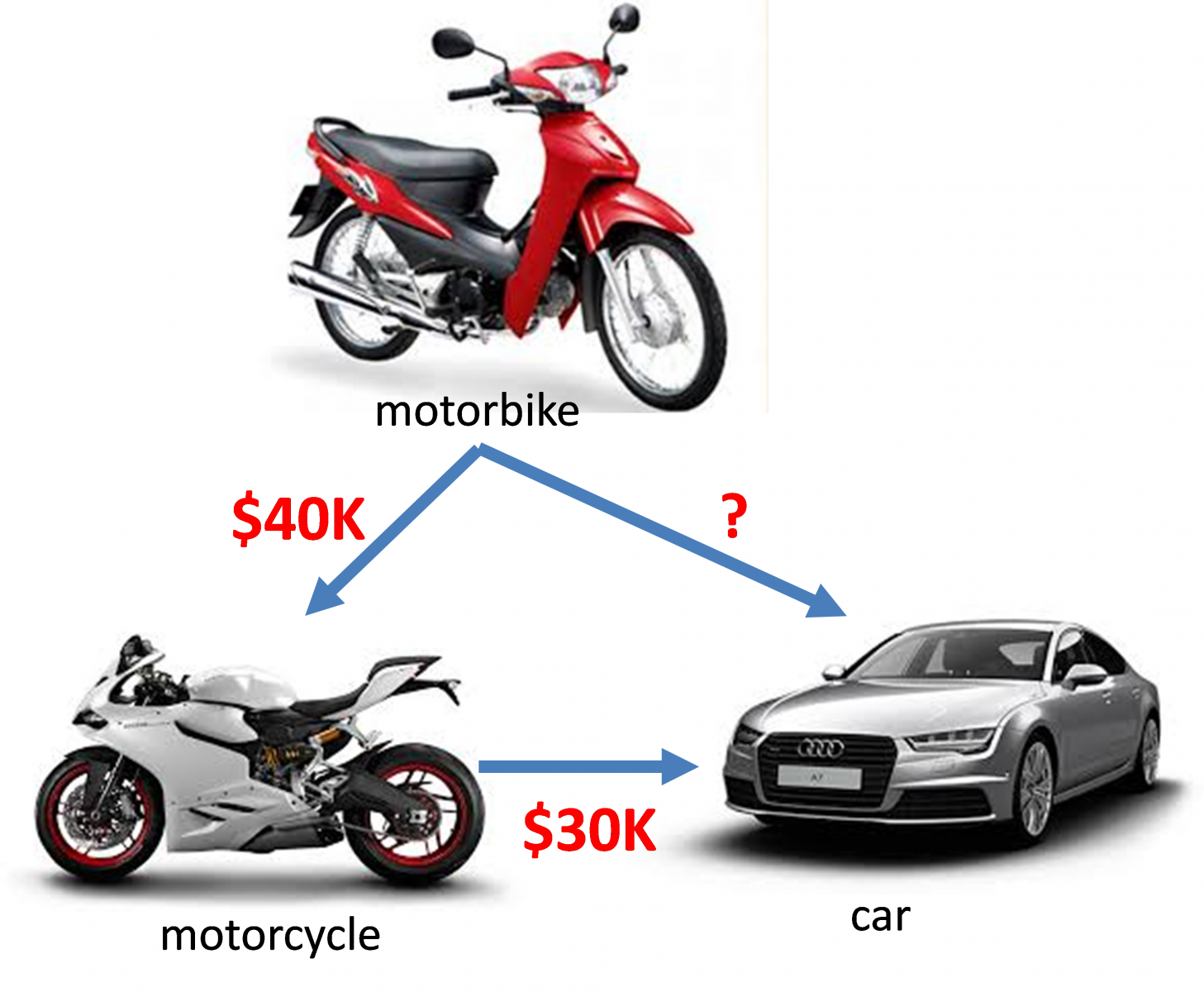
Look at picture below, you have a motorbike. To trade the motorbike for a Ducati motorcycle, you have to give $40K. To trade the Ducati motorcycle for an Audi car, you have to give $30K. So if you want to trade the motorbike for the Audi car, how much money would you need?
Another way of looking at this
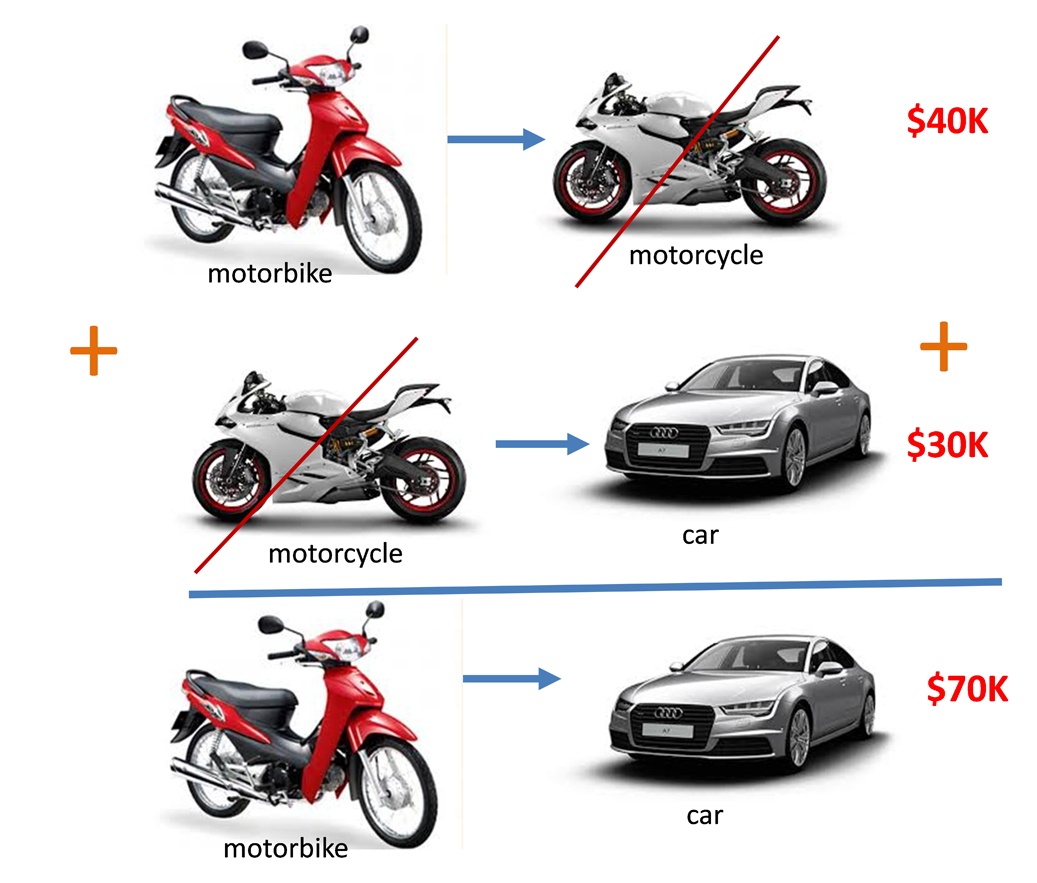
How much money would you get if you trade 2 Audi car for 2 motorbike?
Same same…
First Law of Thermodynamics (Law of Conservation of Energy)
In an isolated system, energy can be transformed but can't be created or destroyed.
Hess's law
Enthalpy change of a reaction is equal the sum of enthalpy change of individual steps