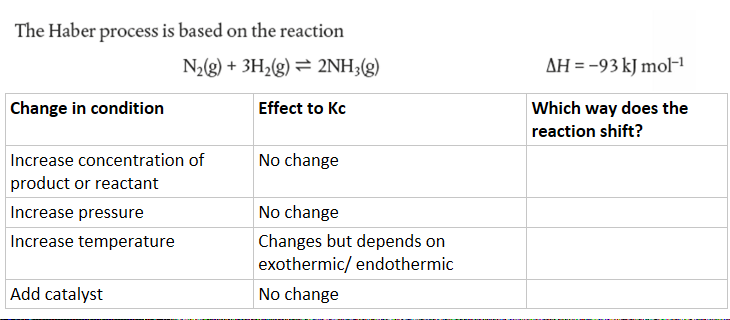
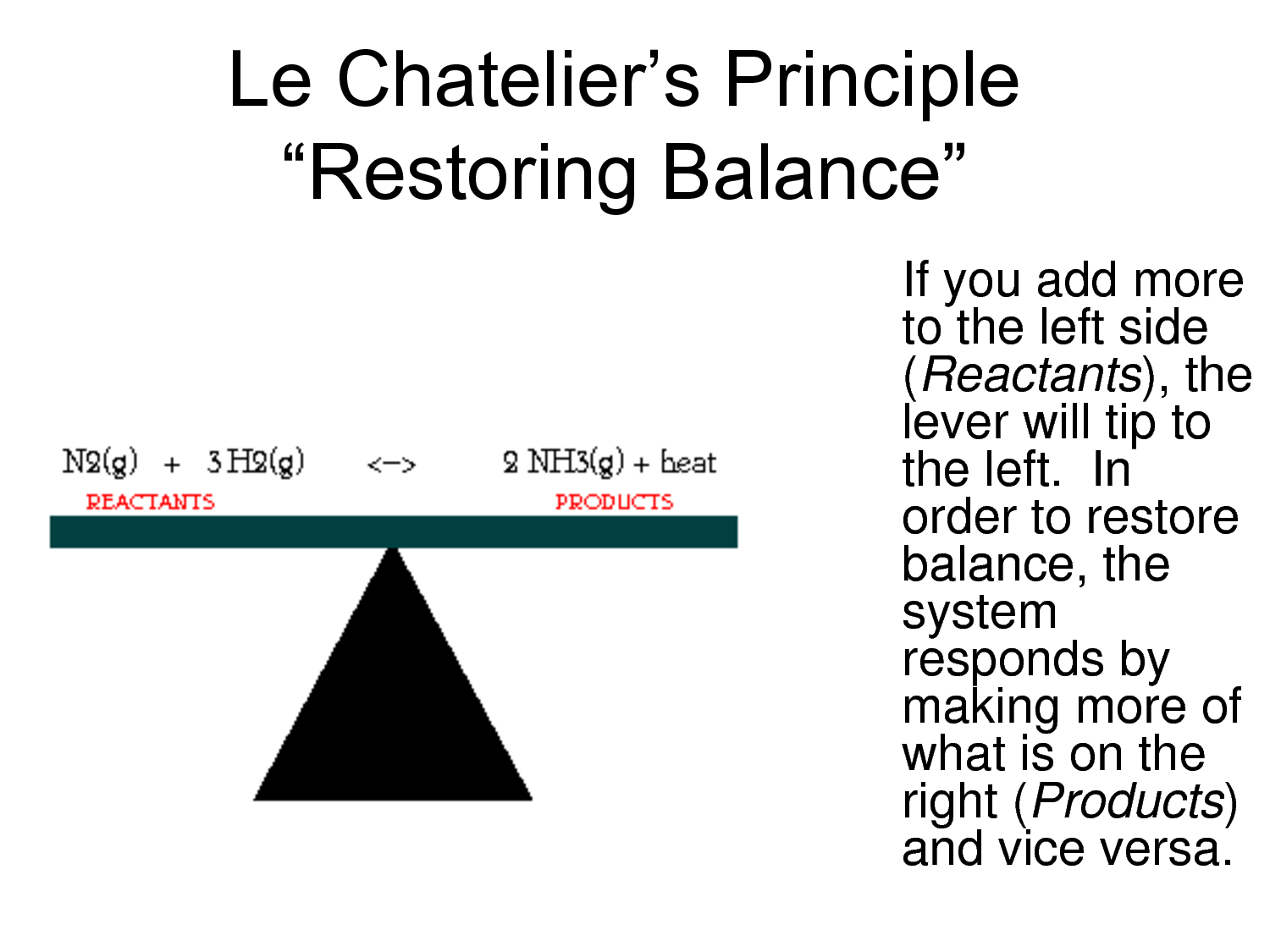
7.1d Le Chatelier's principle
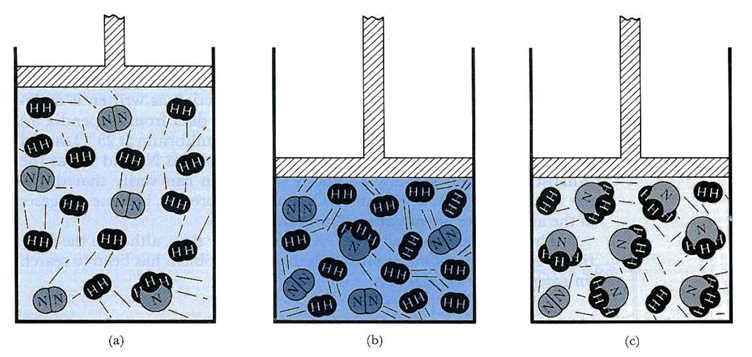
Effect of pressure on reactions on the gas phase depends on the number of moles of gases in the reactants and products
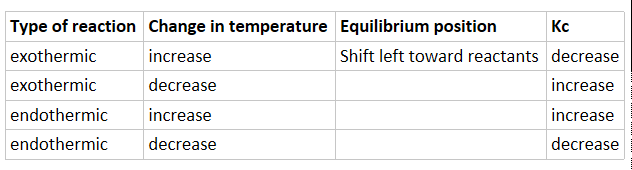
Effect of temperature on equilibrium constant depends on exothermic or endothermic reaction
Effect of catalyst on equilibrium no change to equilibrium, only change to the rate of reaction
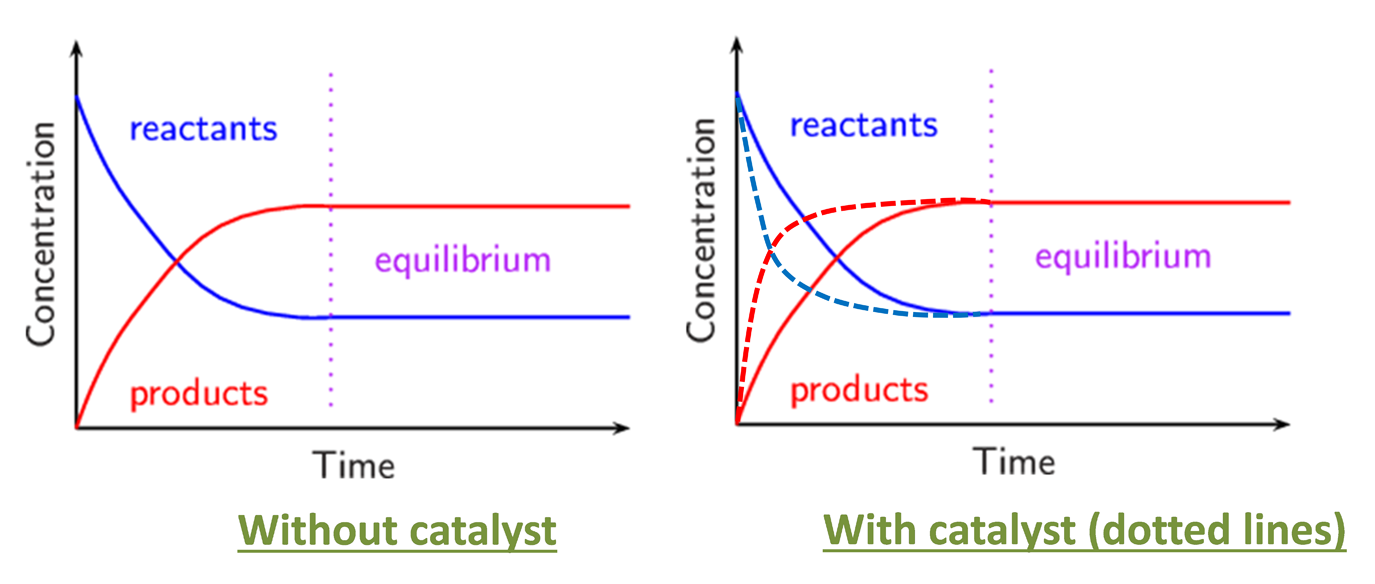
--> How does it applied to the Haber process?
The Haber process is the chemical process to make ammonia, which later used to make explosives or fertilizer.