6.1a Collision theory
Check out this PHET simulation about rates of reaction: Reactions & Rates
Kinetic-molecular theory of gases (aka Kinetic theory of gases)
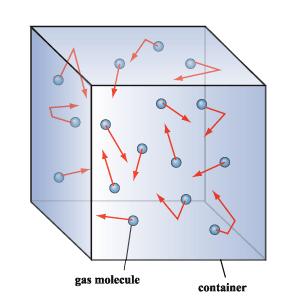
- Gases are large number of particles
- The size of particles are negligible
- Collision of the gas particles are completely elastic
- Average kinetic energy of the particles is proportional to its temperature
In simple words, describe the KMT
Collision theory
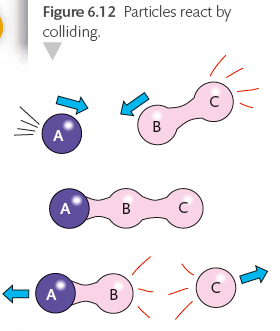
Follow this, explain how temperature determine the rate of chemical reaction









