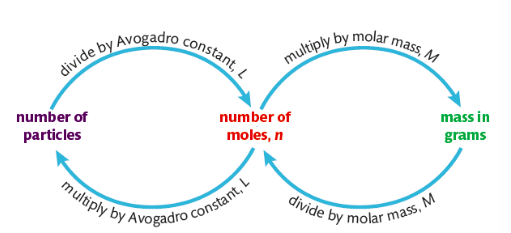
1.2.1 Avogadro number and Molar mass
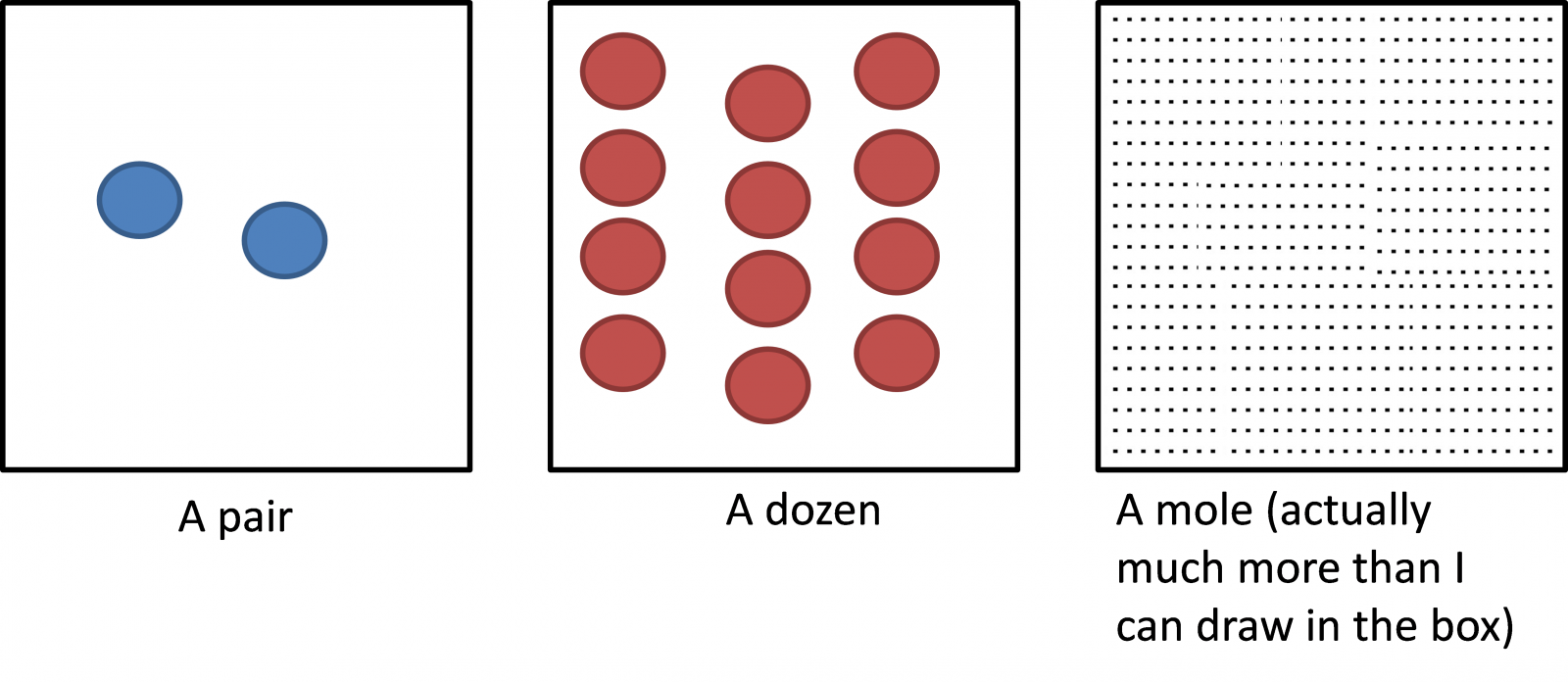
What is a mole?
A very big number of atoms or molecules
called Avogadro number or NA = 6.022 x 1023 mol-1
Why do we use mole as a unit in chemistry?
Look at "how big are things" and the music video below to figure out the answer to this question.
What is Molar mass?
- The mass of one mole of atom. Example: 1 mole of Carbon has a mass of 12.011 g. Thus, Carbon molar mass is 12.011 g/mol
- Molar mass, M, has the units g mol-1. This is the same as g/mol
Doing calculation with mole