1.2.2 Definitions of fomula
What is the difference between empirical, molecular and structural formulas?
Ie. What information can be gained from each type of formula?
Empirical formula: gives the simplest whole ratio of the actual number of atoms present in one molecule of the compound
Eg. CH2O
Molecular formula: gives the actual number of atoms present in one molecule of the compound
Eg. C3H6O2
Structural formula: At least three different possible compounds have the above empirical and molecular formula (there are other possibilities):
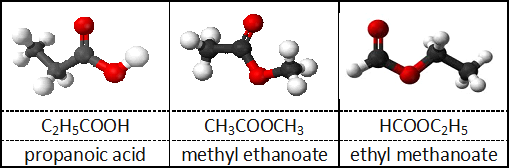
Formula with a dot: Sometimes molecules are connected to water (or other ligands, more on this in later unit) and the connection is represented with a dot.
Example: hydrated iron(II) ammonium sulfate, (NH4)2 Fe(SO4)2 •6H2O(s)
Scary as it seems, this formula means 6 water molecules are connected to 2 ions of NH4 , 1 Fe ion, and 2 SO4 ions









