1.2.3 Percentage composition
Example:
Determine the empirical formula of an organic compound that contains 75% carbon and 25% hydrogen by mass.
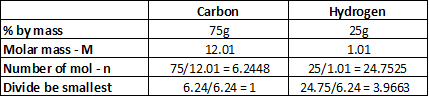
The ratio C:H is 1:4
Empirical formula CH4
Determine the molecular formula if the molar mass of this organic compound is 32 g/mol.
If it was CH4, the molar mass is 12.01 + 4* 1.01 = 16.05
Times over 32/16.01 = 1.9988
Molecular formula C2H8









