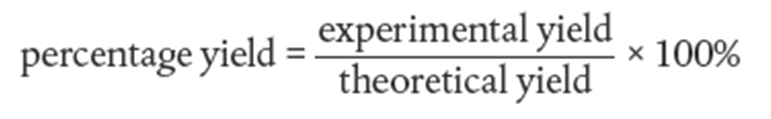1.3.1 Limiting reactants and yield
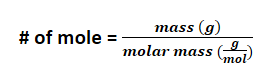
Reactants can either be a limitting reagent or an excess:
- The limiting reagent (reactant) is used up completely in a chemical reaction
- The excess is the reactant that will be left over once the reaction is completed.
How to do calculations with moles and masses?
masses varies with the compounds thus you must do RATIO with the number of moles. The calculated amount that you expect is also called "theoretical yield"
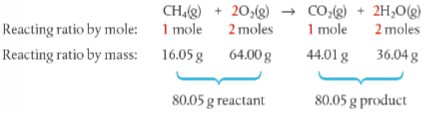

What is the difference between limiting reactant and excessed reactant?
Prepare and have students do the experiment below to answer this question
Experiment about limiting reactant
How do you calculate percentage yield?