1.3.2 Moles and gases
Ideal gas law
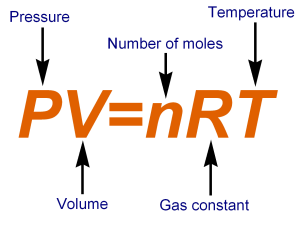
Make sure you use the appropriate pair of PV units (Eg. kPa must be with dm3) and always use Kevin for temperature.
K = oC + 273 Eg. 0oC is 273K
2 assumptions that are NOT true when dealing with real gas (instead of ideal gas):
when describing an ideal gas with kinetic theory we assumes this but it is not true for real gases at high temperature and pressure
- The volume of the gas particles is negligible
*At low pressure this is negligible but at high pressure is can be about 20% of the total volume
- The are no attractive forces between the particles
*at low pressure the particles are so widely spaced that interactive forces are highly unlikely but increases at higher pressures
*low temperatures slow particle movement and so increase interactive forces
1. At STP (standard temperature and pressure), what is the volume of 1 mole of gas?
Don't memorize this, use the data booklet. The molar volume is 22.7 dm3.
2. For reaction with gases, ratio can be done using the volume
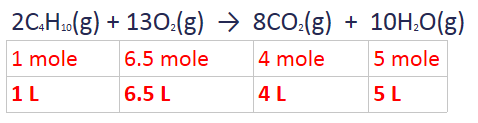
Example
Nitrogen gas (N2) can be prepared from this reaction:
2NH3 + 3CuO → N2 + 3Cu + 3H2O
If 18.1L NH3 are reacted with 90.40 g CuO, determine the volume of N2 that can be formed at STP.
A bit of history should you interested in which scientist figured out which part of the ideal gas equation...









