1.3.3 Moles and solutions
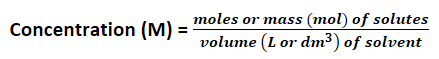
Note: another unit for concentration g/L
- Use square brackets to show concentration, eg [HCl] = 2.0 M
- 1 L = 1 dm3
1 mL = 1 cm3
1 L = 1000 mL - Must find the number of moles first before doing ratio (similar to the other calculation)
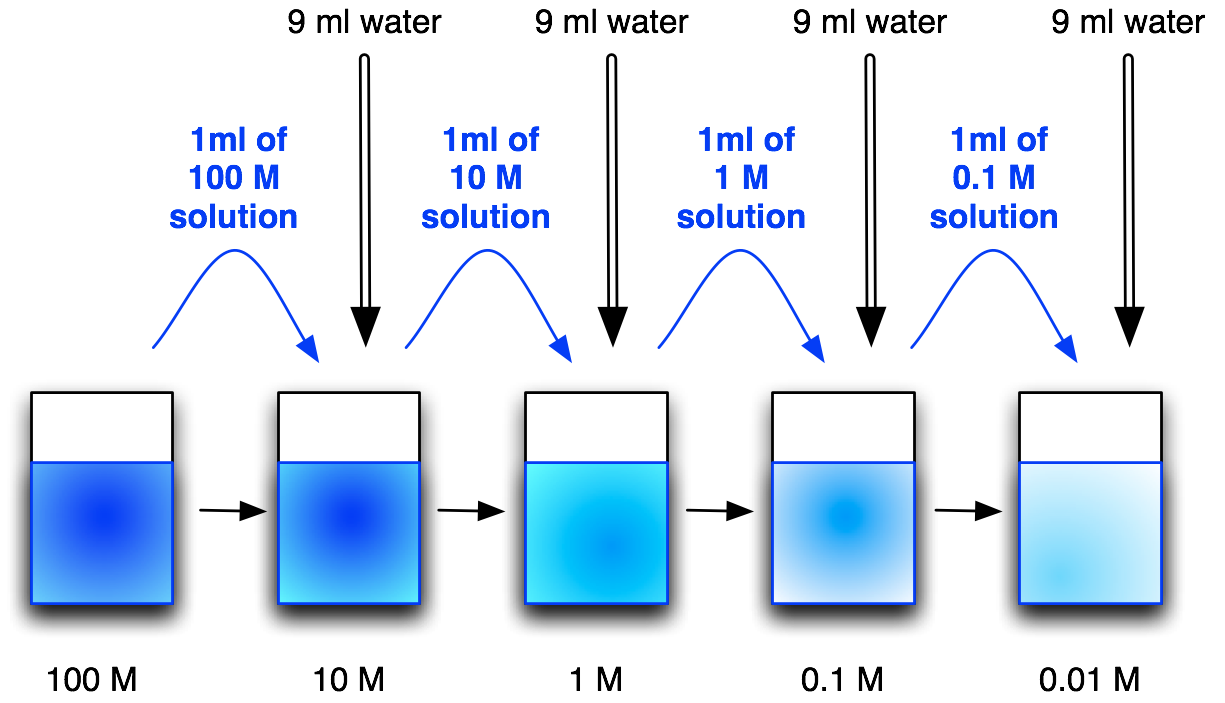
Dilute a solution
- To make a dilution is to make a smaller concentration from a more concentrated solution (stock solution) by adding solvent.
- Serial dilution is to dilute multiple times