2.2b Wave spectrum
Electromagnetic spectrum - Data booklet p.3
Vocab:
- Electromagnetic = any wave that is both electric and magnetic such as light, microwave, radio wave, UV…
- Spectra (plural), spectrum (singular) = a range of something. In this case, a range of different waves.
- Emission (n.), emit (v.) = give out.
- Absorption (n.), absorb (v.) = take in
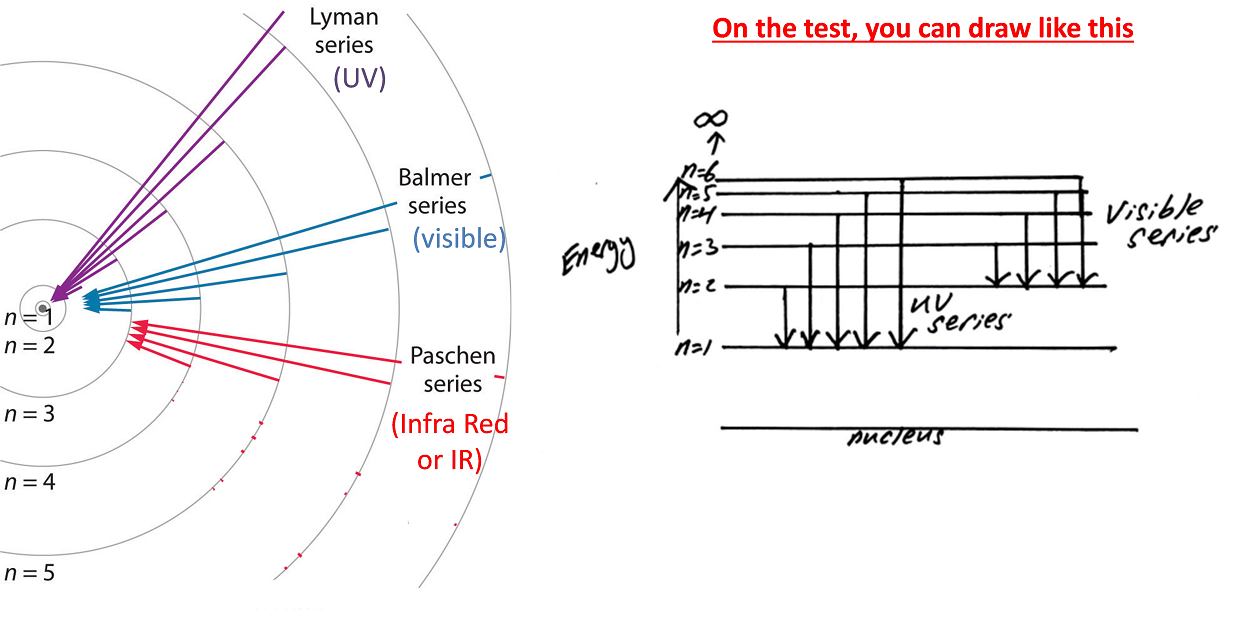
Note: electromagnetic wave are quantized, meaning that they come in discrete amount of energy (quanta). Thus, an electron can be on n=1 or n=2, but cannot be on n=1.5 --> new addition
- Excited electron: When an electron jump up from a lower energy level to a higher energy level, it has to absorb an amount of energy that equals the gap between the levels. The lower level is called ground state, the higher level is excited state. See the reason in "quantized" above.
- Photon emission: When an electron drop down from an excited state to a lower energy level, it will emit a photon (ie. a unit of light) that has the same energy as the gap between the levels. Again quantized.
- The equation to measure such energy of a wave (= photon) is in Data Booklet. Know the relationships, SL don’t need to do # problems.
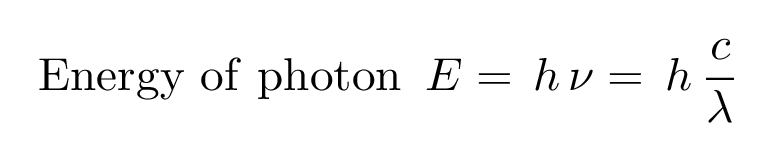
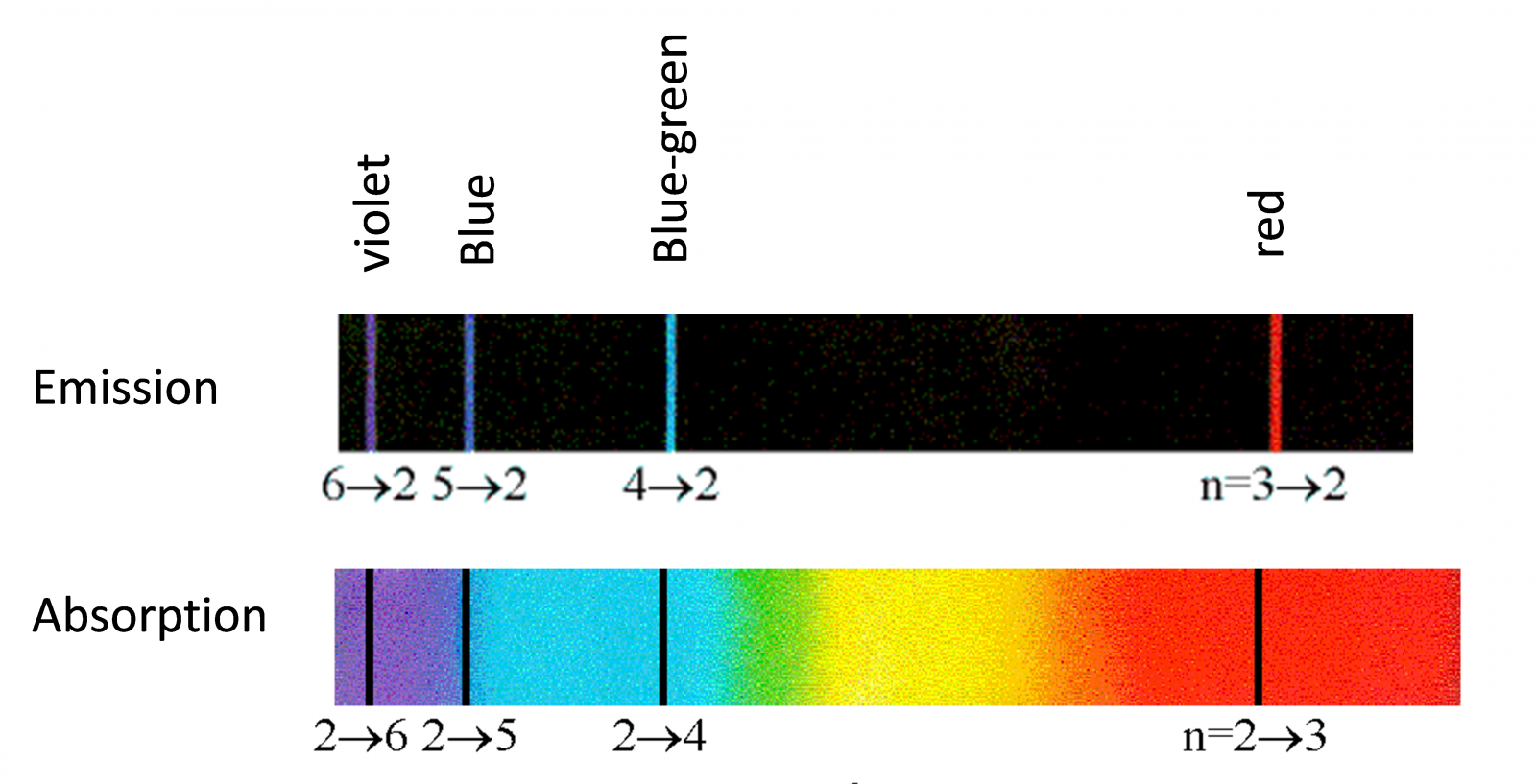
Example: the visible spectrum of Hydrogen atom (also known as Balmer series).
Note that there are other series for Hydrogen too, but the waves are NOT visible like UV or Infra Red light, see below.