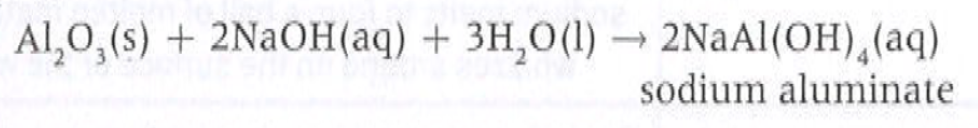3.2a Characteristic of ions
1. Ionic radius
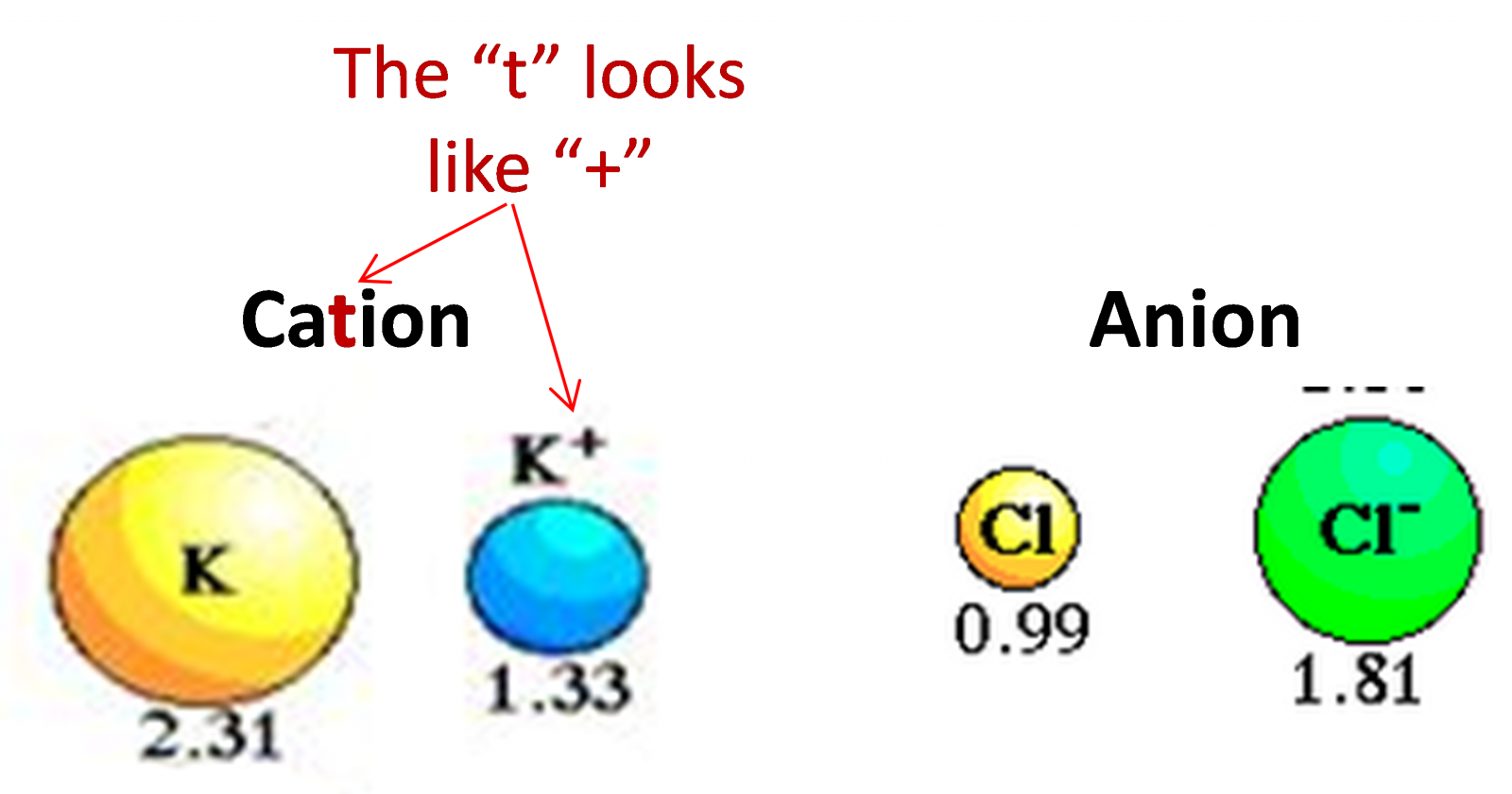
Follow the logic:
Metal (left side of periodic table) = low electronegativity = lose electrons = Cation = oxidized
Non-metal is the opposite of metal = Anion = reduced (because the atom goes from 0 charge to -1, -2, -3… the number is lowered or reduced)
The wanna-be-Noble rule: everything wants to lose or gain the appropriate numbers of electrons to be like Noble gas. Why?
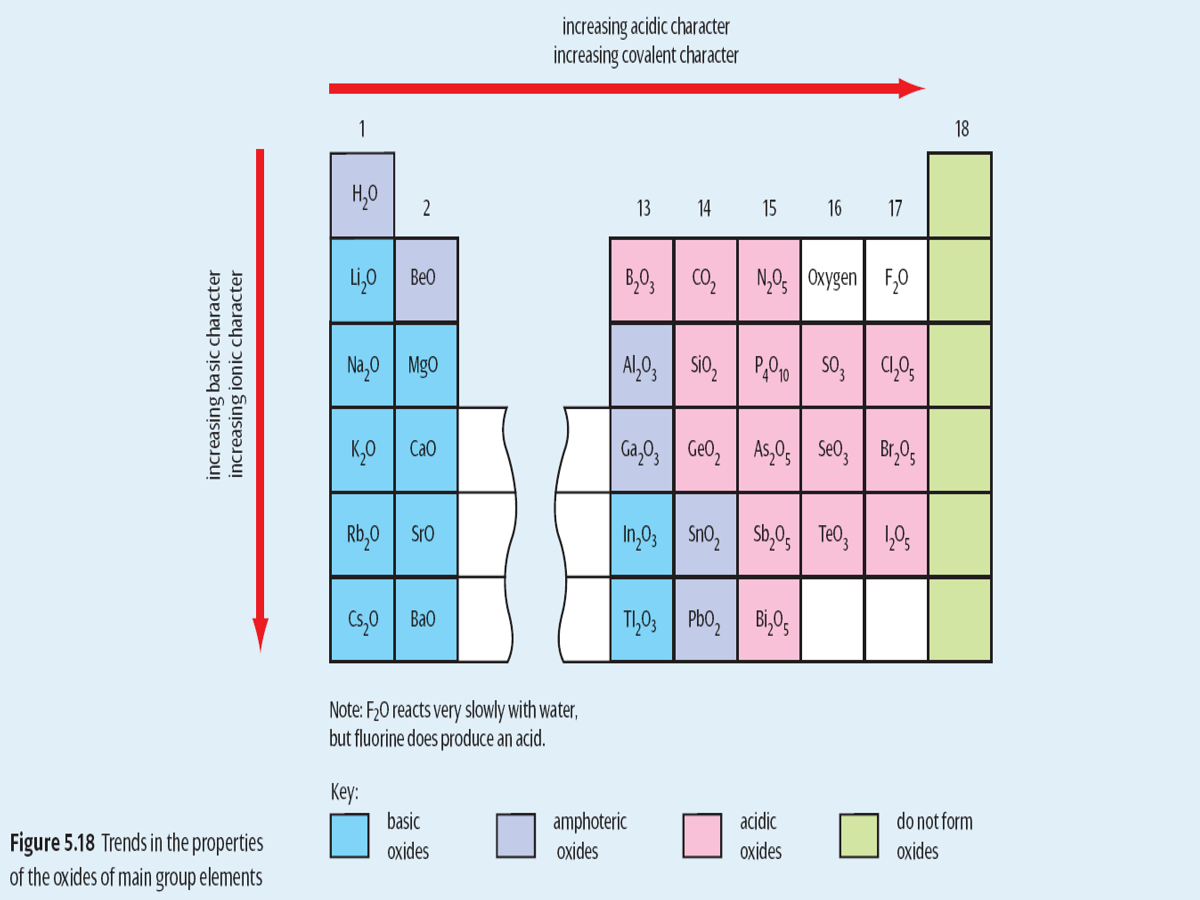
2. Acidic, basic or amphoteric oxide in water
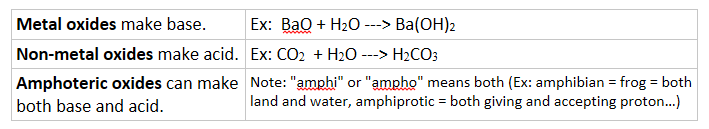
Example of amphoteric oxide Al2O3
Acting as an acid