9.1a Oxidation state and naming
Definition of oxidation and reduction

Oxidation state
an increase in oxidation number represents oxidation, and a decrease in oxidation number represents reduction
Example:
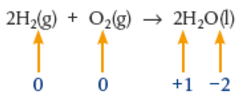
Hydrogen has been oxidised (oxidation state has increased from 0 to 1) and oxygen has been reduced (oxidation state decreased from 0 to -2)
Exceptions you have to memorize
O is always -2 except for in H2O2
H is always +1 except for in NaH and similar compounds where H goes at the end
Note that in an ionic compound, the + ion is always on the left, - ion is on the right
Naming of metal using oxidation state
- Some elements exhibit different oxidation states so sometimes this information is used in the name of the compound
- Roman numerals that correspond to the oxidation number are used in brackets after the name of the element, eg copper(I) oxide and copper(II) oxide
Example:
![]()
![]()









