9.1d Activity series
Activity series show oxidizing power (so you know which way the electrons are moving)
This is in data booklet page 24
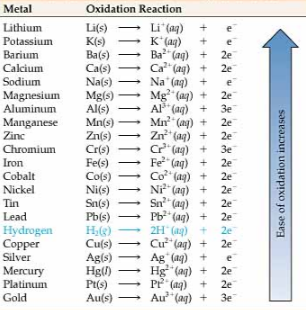
Example:
If a piece of Zinc and a piece of Copper are put together, the piece of Zinc will oxidize = lose electron to the piece of Copper. Why? Think back to Electron affinity Periodicity unit
More on this when we look at voltaic vs. electrolytic cells









