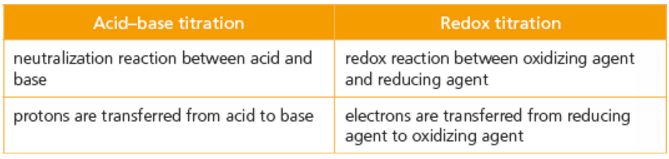
Redox titration
There are a number of redox titration, they all rely on a change in color due to a change in oxidation state (Note that different oxidation states for transition metal give different color)
Key point: after balancing the reaction, look at the coefficient of the titrant (in the burette) and that of the titrated (in the flask)
Use the coefficient for calculation of the unknown mole.
If the ratio is 1:1, you can use the c1 x V1 = c2 x V2
The Winkler method is an example of redox titration

The amount of dissolved oxygen is traditionally determined using the Winkler method. This was devised by the Hungarian Lajos Winkler (1863-1939) for his Ph.D. thesis in 1888. Excess manganese(II) sulfate and hydroxide ions are added to a known volume of the water sample. The dissolved oxygen oxidises the manganese(II) ions to give a brown precipitate (usually considered to be a hydrated form of manganese(IV) oxide). In the presence of acid this then oxidises iodide ions into iodine and is itself reduced back to manganese(II). The iodine produced is titrated with standard sodium thiosulfate solution using starch as the indicator.
2Mn2+(aq) + O2(aq) + 4OH− (aq) → 2MnO2(s) + 2H2O(l)
MnO2(s) + 2I−(aq) + 4H+(aq)→ Mn2+(aq) + I2(aq) + 2H2O(l)
2S2O32−(aq) + I2(aq) → S4O62−(aq) + 2I−(aq)
By following the stoichiometry it can be seen that one mole of dissolved oxygen is equivalent to four moles of thiosulfate ions.
This method demonstrate a redox titration
redox titrations are used to determine the unknown concentration of a substance in solution
- winkler method









