9.2a Voltaic vs. electrolytic cells
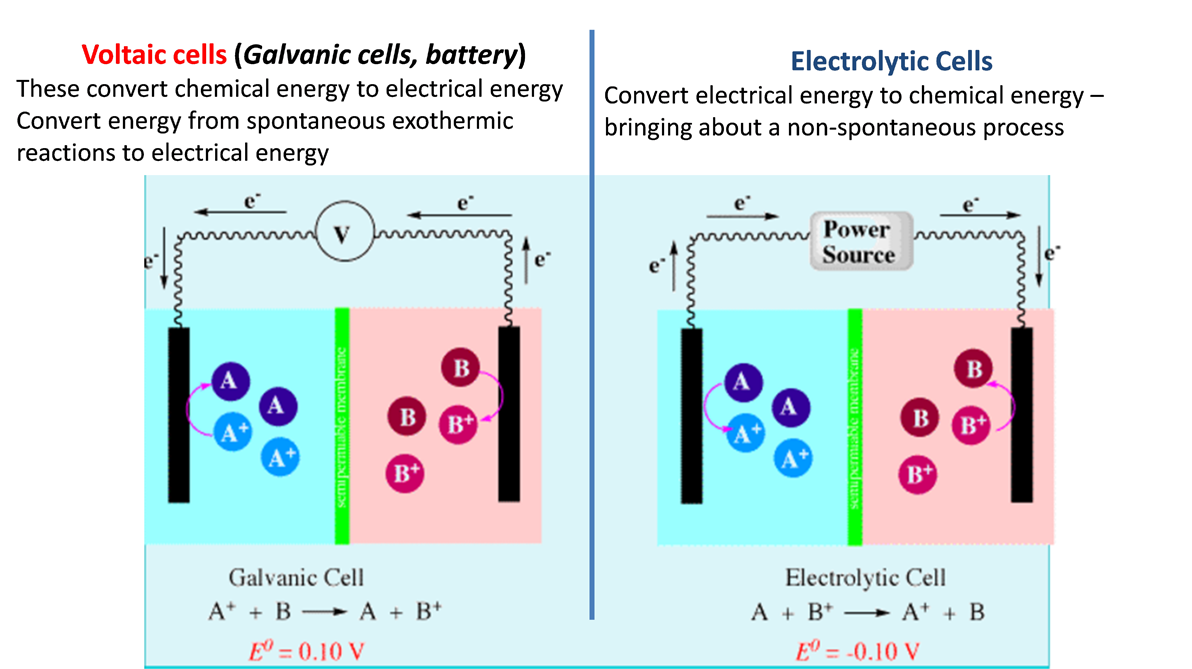
What are the differences?
- Salt bridge:
- Power source:
- The charges of the anode and cathode:
Cell diagram
Cu(s) | Cu2+(aq) || Ag+(aq) | Ag(s)
Remember that Oxidation always on the left, then Reduction on the right
Half-cell notation:
–Different phases are separated by vertical lines
–Species in the same phase are separated by commas
Special cases:
1. when the species are all gases and aqueous (no solid), you need an inert electrode (Pt (s)) to connect the wire to. Therefore, another vertical line.
2. Electrodes involving metals and their slightly soluble salts, add another line.









