9.2b How to draw?
How do you draw voltaic (galvanic) cell and electrolytic cell?
Three things that are always true for both cells
1. AnOx and RedCat
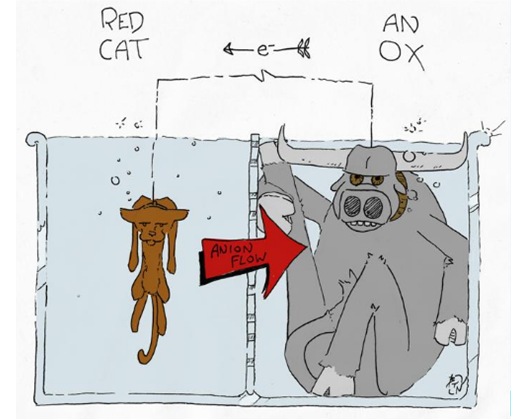
2. Oil Rig
Oxidize Is Lose (electron), Reduction Is Gain (electron)
3. Current flow from to Cathode (red) to Anode (oxi) - IB physics
Note that this is a convention. When scientist discover electricity they didn’t know that electricity is the flow of electrons. Thus, they just randomly assign the current flow to be from cathode to anode
Electron flow from Anode (oxi) to Cathode (red) - IB chemistry
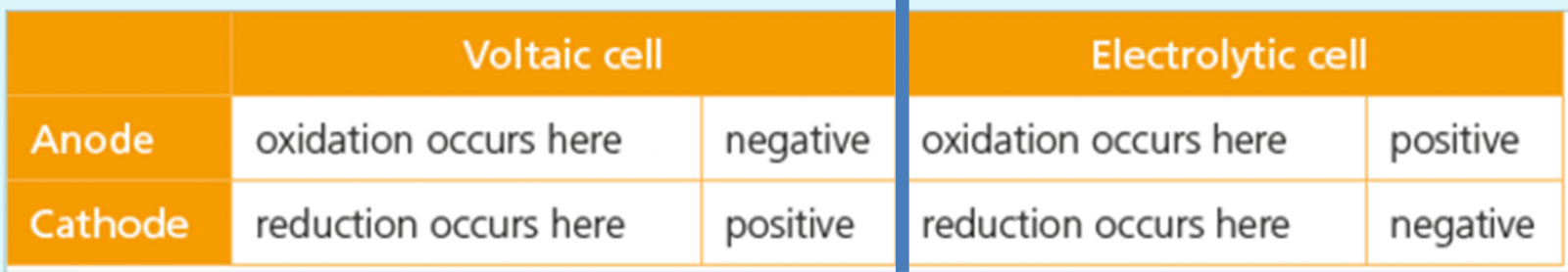
What about the charges? Remember that voltaic and electrolytic are opposite of each other
How do I know which element is oxidizing, which one is reducing?
Use the data booklet table 25
- Voltaic cell: the more reactive (on top) will oxidize
- Electrolytic cell: the electrode that receives the electrons from the power source will reduce regardless of oxidizing power. This is because you are putting energy into driving the electrons.
Or use table 24

how to draw voltaic and electrolytic cells









